- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The MIN6 mouse insulinoma cell line is derived from an insulinoma tissue developed in a transgenic mouse in which the human insulin promoter drives the expression of the Simian Virus 40 Large T antigen. In comparison to primary pancreatic beta cells, MIN6 mouse insulinoma cell line grows more robustly and more vigorously in cell culture, while retaining important features of beta cells, such as the exclusive expression of the so-called "liver-type" glucose transporter (Glut2) and their ability to modulate insulin secretion in response to changes in glucose concentration in the growth medium, and it has been a staple strain in diabetes research since its establishment in 1990. In addition, the expression of the major histocompatibility complex I (MHC I) is lacking in MIN6, as in the non-diabetic beta cells, but can be induced by proinflammatory cytokines, which would render them susceptible to T-cell mediated cytotoxicity. This closely mimics the behavior of beta cells isolated from diabetic tissues, where chronic inflammation is commonly observed, and it has made MIN6 an attractive cell line model for investigating the mechanism of beta cell loss due to their autoimmune destruction.
Nickel Sulfate Induced GSIS Injury in MIN6 Cells by Activating the JNK Pathway Through Oxidative Stress
Nickel has an impact on human health, especially in the context of new energy industries. Nickel's influence on glycemia remains controversial, and the effect and mechanism of nickel on islet function still need further exploration.
MIN6 cells were treated with different concentrations of nickel sulfate (NiSO4) (0, 75, 150, and 300 µg/mL) for different durations (0, 12, 24, and 48 h). The study measured cell cycle progression, apoptosis, reactive oxygen species (ROS) production, oxidative stress–related indexes (T-SOD, TBARS, 8-OHdG, and GSH), glucose-induced insulin secretion (GSIS), and the expression of JNK pathway–related proteins, pancreaticoduodenal homeobox-1 (PDX-1), glucose transporter 2 (GLUT2), and forkhead box protein O1 (FOXO1). NiSO4 damaged MIN6 cells in a time- and dose-dependent manner. NiSO4 blocked the cell cycle, induced apoptosis, and reduced insulin secretion in the GSIS experiment. NiSO4 also induced ROS production, increased oxidative stress–related indexes (TRABS and 8-OHdG), and decreased antioxidant stress–related indexes (GSH and T-SOD). In addition, NiSO4 activated the JNK pathway, upregulated FOXO1 protein expression, and inhibited PDX-1 and GLUT2 protein expression, affecting insulin release during GSIS.
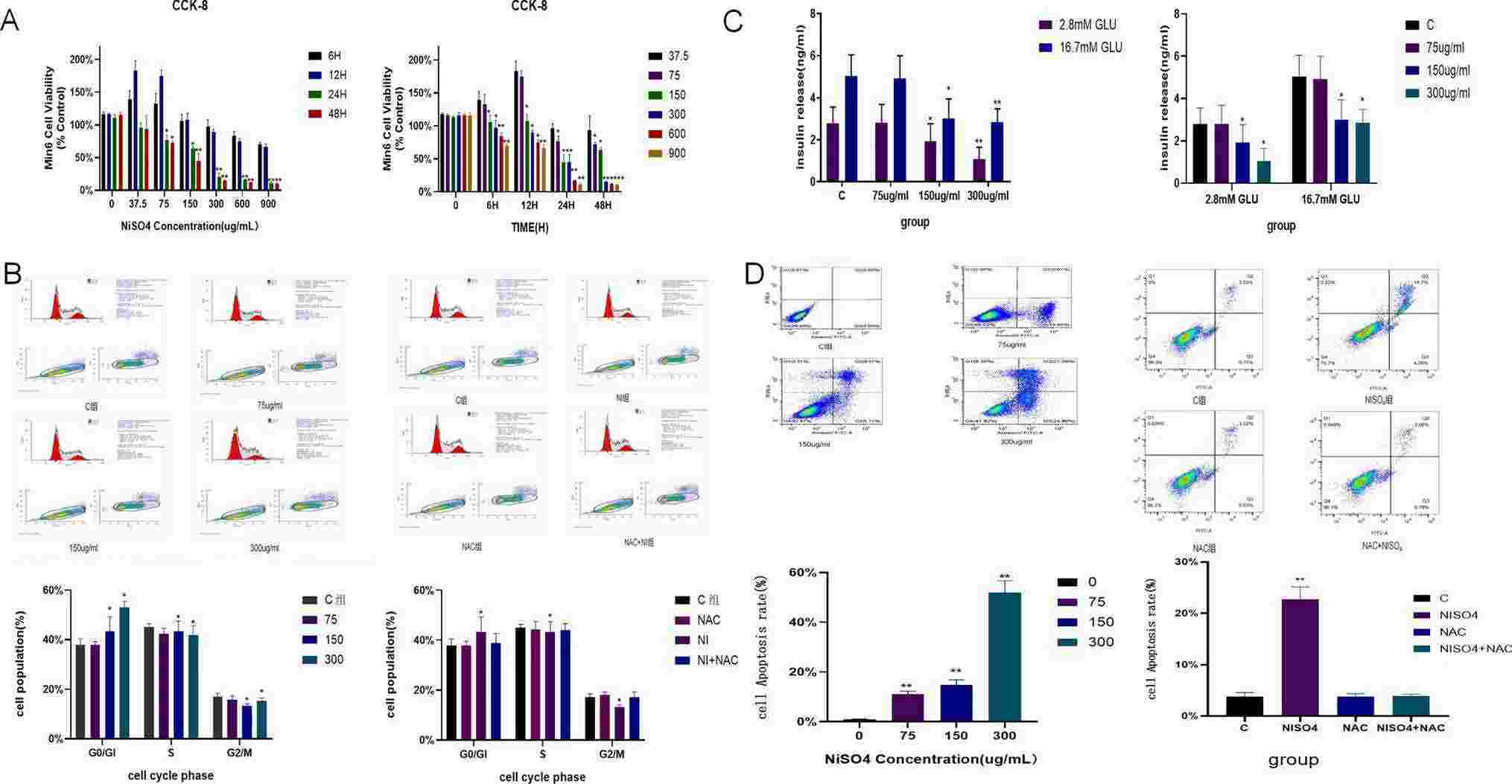 Fig. 1. NiSO4 reduced the viability of MIN6 cells, blocked the cell cycle in the G0/G1 phase, induced cell apoptosis, and caused damage to the function of islet cells (Sun, Bo, and Hui Chen. 2024).
Fig. 1. NiSO4 reduced the viability of MIN6 cells, blocked the cell cycle in the G0/G1 phase, induced cell apoptosis, and caused damage to the function of islet cells (Sun, Bo, and Hui Chen. 2024).
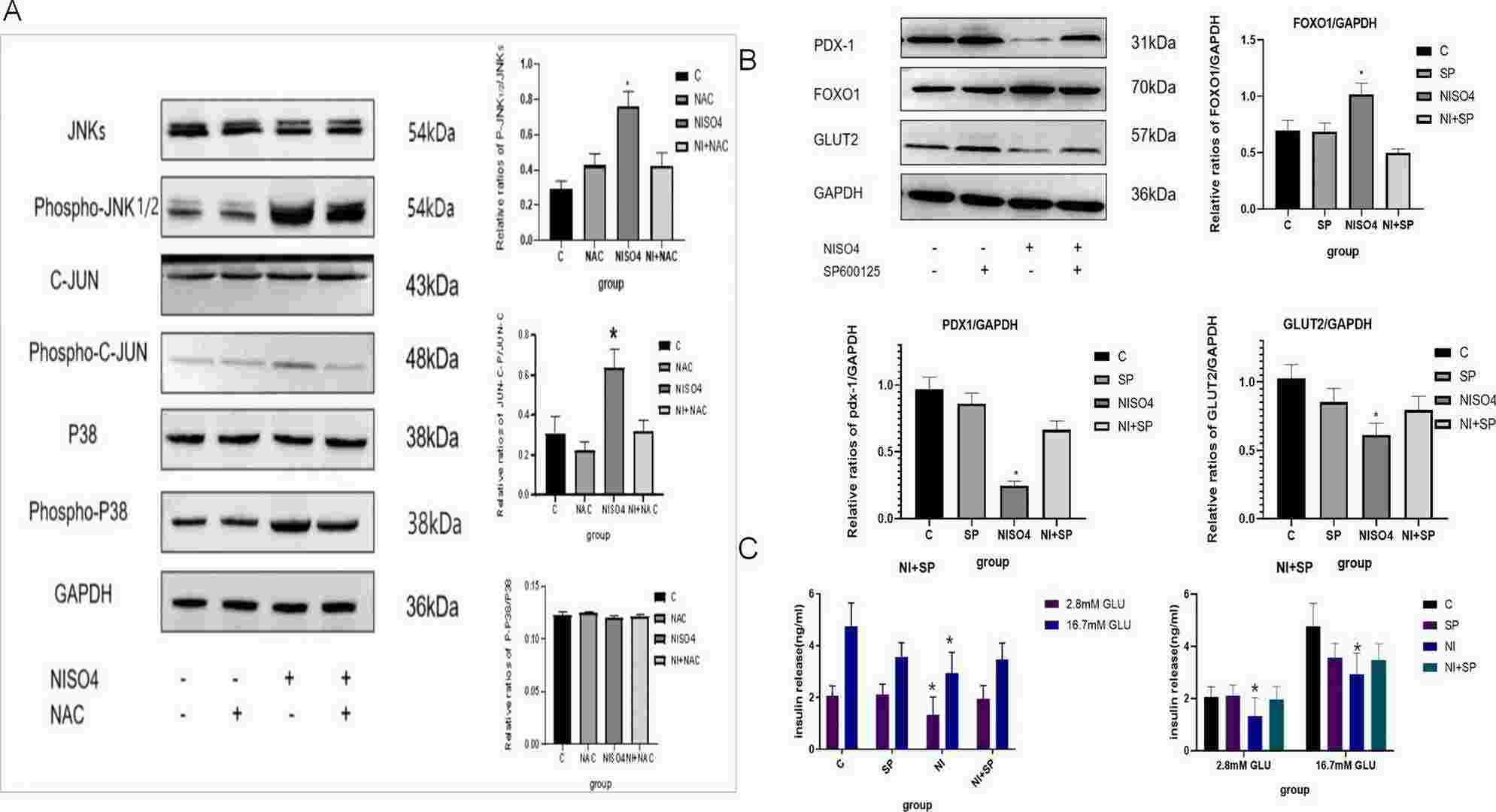 Fig. 2. NiSO4 activated the JNK pathway, upregulated FOXO1 protein expression, and inhibited PDX-1 and GLUT2 protein expression, resulting in decreased GSIS function of MIN6 cells (Sun, Bo, and Hui Chen. 2024).
Fig. 2. NiSO4 activated the JNK pathway, upregulated FOXO1 protein expression, and inhibited PDX-1 and GLUT2 protein expression, resulting in decreased GSIS function of MIN6 cells (Sun, Bo, and Hui Chen. 2024).
Puerarin Ameliorates High Glucose-Induced MIN6 Cell Injury by Activating PINK1/Parkin-Mediated Mitochondrial Autophagy
The dysfunction of pancreatic β-cells plays a key role in the pathogenesis of type 2 diabetes mellitus (T2DM). Despite numerous studies have demonstrated the anti-inflammatory and antioxidant properties of puerarin, the protective effects of puerarin on β-cells remain poorly understood. Hence, this study aimed to explore the effects of puerarin on β-cell dysfunction in a hyperglycemic environment via the PINK/Parkin-mediated mitochondrial autophagy pathway.
MIN6 cells were exposed to glucose concentrations of 5 mM, 10 mM, 20 mM, and 30 mM for 24 h, 48 h, and 72 h, respectively. Results indicated a significant decrease in MIN6 cell viability following 48 h of exposure to 30 mM glucose concentration. Puerarin intervention markedly attenuated ROS production, restored mitochondrial membrane potential, induced PINK/Parkin-mediated mitochondrial autophagy, suppressed activation of the mitochondrial apoptotic pathway, mitigated apoptosis, and enhanced insulin secretion in a high glucose (HG) environment. The findings of this investigation contribute to a deeper understanding of the precise mechanism underlying the protective effects of puerarin on β-cells and offer a theoretical foundation for advancing puerarin-based therapeutics aimed at ameliorating T2DM.
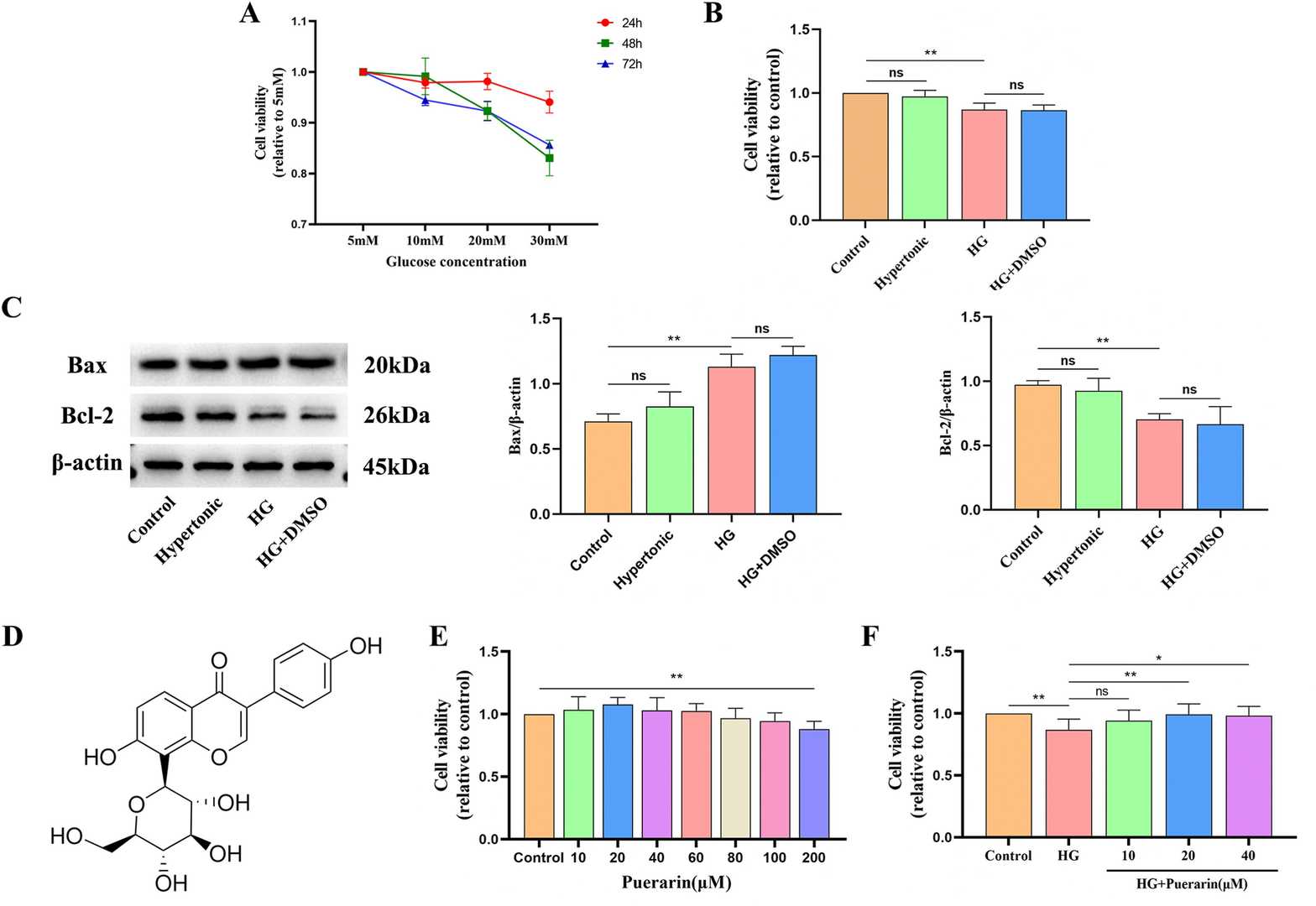 Fig. 3. Effect of different concentrations of glucose and time on the viability values of MIN6 cells (Zhu, Hongyang, et al. 2024).
Fig. 3. Effect of different concentrations of glucose and time on the viability values of MIN6 cells (Zhu, Hongyang, et al. 2024).
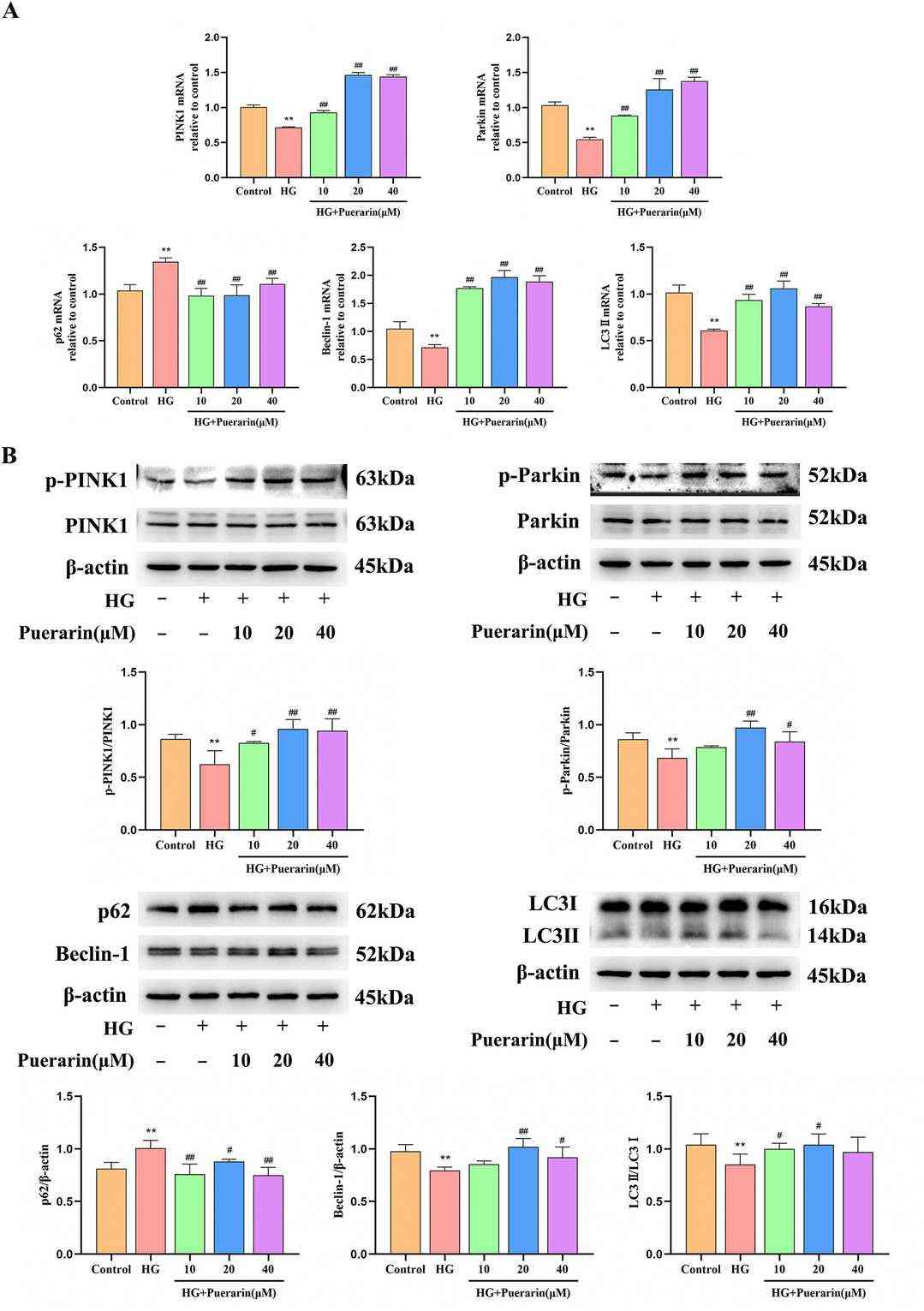 Fig. 4. Puerarin enhances PINK1/Parkin-mediated mitochondrial autophagy in HG-induced MIN6 cells (Zhu, Hongyang, et al. 2024).
Fig. 4. Puerarin enhances PINK1/Parkin-mediated mitochondrial autophagy in HG-induced MIN6 cells (Zhu, Hongyang, et al. 2024).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells