Mastering Cell Culture and Cryopreservation: Key Strategies for Optimal Cell Viability and Stability
Cell Culture
Once a cell line is established, optimizing culture conditions and expansion strategies is crucial for achieving rapid proliferation while maintaining its biological characteristics and stability. The following are key points in cell culture and expansion:
(1) Culture media selection:
Choose an appropriate basal medium according to the cell type and requirements. Special media formulations, such as serum-free, protein, and peptide-based media, may be necessary for specific cell types.
(2) Media components optimization:
The media composition needs adjustment to supply proper levels of glucose, amino acids and vitamins which support both cell growth and their correct functioning.
- Cell proliferation and differentiation rely on specific functions preserved by adding growth factors along with hormones and cytokines.
- Continuously monitor and improve the media formulation to match the evolving cell growth states.
(3) Temperature and humidity control:
The incubator temperature should remain at 37°C while relative humidity levels must be kept above 95%.
(4) PH and ionic balance maintenance:
- For optimal cell growth conditions, you need to frequently monitor media pH values and maintain them between 7.2 and 7.4.
- Keep track of calcium and magnesium levels in the media, and maintain their concentrations within acceptable limits.
(5) Passaging:
- Intermittent passaging: Once cells reach a certain density, transfer them to new culture vessels through digestion and separation to increase cell quantity.
- Continuous passaging: For cells requiring long-term culture, implement continuous passaging by regularly separating and transferring cells.
(6) Expansion culture vessel selection:
- Culture dishes: Suitable for small-scale cell culture, such as experimental studies and preliminary screenings.
- Cell flasks and culture bags: Appropriate for large-scale cell expansion, such as in the production of bioproducts and cell therapy products.
(7) Centrifugation and aliquoting:
- Centrifugation: Use a centrifuge to separate and collect cells from the culture for subsequent processing and analysis.
- Cell aliquoting: Dispense collected cells as needed, storing them at -80°C or in liquid nitrogen for future use.
Quality Control in Cell Culture
- Quality control is key to ensuring the reliability of results in cell-based experiments. The quality control of cells typically includes the following aspects:
- Monitoring Cell Viability: Regularly assess cell viability using assays such as MTT or CCK-8 kits to evaluate cell proliferation capability.
- Observation of Cell Morphology: Use microscopy to observe cell morphology. Normal cells should exhibit good adherence and characteristic morphological features.
- Contamination Detection: Regularly test for mycoplasma contamination, utilizing PCR or fluorescent staining methods for detection.
Cell Cryopreservation
Why cryopreserve cells?
1) Temporarily halts cellular growth to maintain their original characteristics, allowing for revival and experimental use when needed.
2) Mitigates genetic changes that may occur due to continuous growth and passaging.
3) Ensures stable cell lines for reproducible results over an extended period, protecting against loss due to contamination or unforeseen events during cultivation.
4) Allows for the secure and convenient shipment of cell lines.
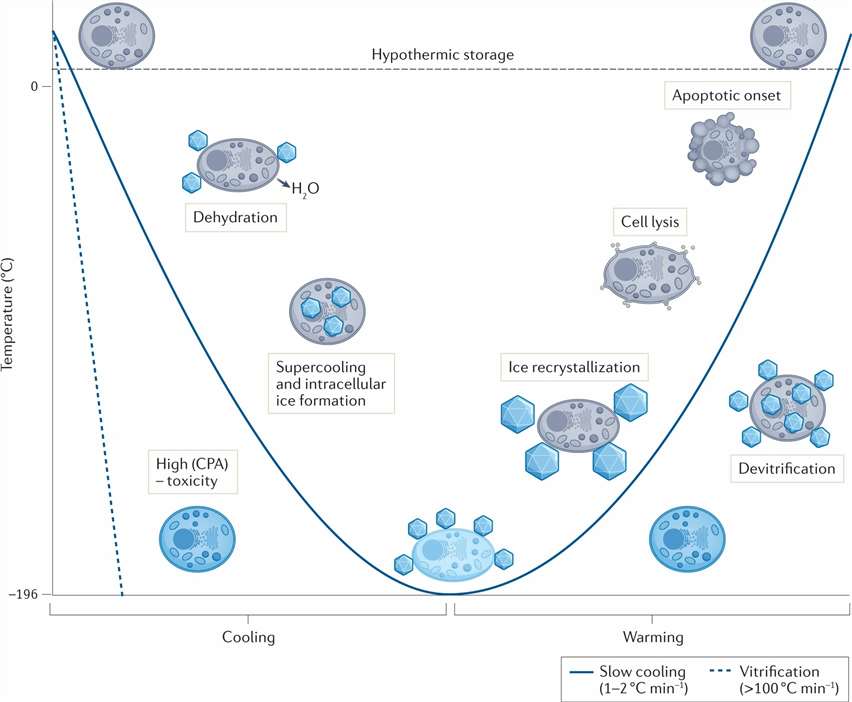 Fig. 1. Potential mechanisms of cellular damage during cryopreservation (Murray KA, Gibson MI. et al., 2022).
Fig. 1. Potential mechanisms of cellular damage during cryopreservation (Murray KA, Gibson MI. et al., 2022).
Key points in cell cryopreservation
Preparation Before Freezing
- Preparation before freezing is crucial for cell viability and the conditions following thawing.
- Collection and Counting of Cells: Collect cells during the logarithmic growth phase through trypsin digestion. Cell numbers should be quantified using hemocytometer counting or flow cytometry techniques.
- Assessment of Cell Viability: Use trypan blue staining or fluorescent dyes like PI (propidium iodide) to measure cell viability and confirm it exceeds 90% before freezing.
Cryoprotective Agents
- Cryoprotective agents play a crucial role in protecting cells from ice crystal damage during low-temperature storage.
- DMSO (dimethyl sulfoxide) and glycerol make up the standard cryoprotectants. Researchers typically use DMSO in a 10% concentration and glycerol at an approximate concentration of 20%.
- After adding cryoprotectants, cells need to equilibrate for 10-30 minutes at 4°C. Cells that absorb cryoprotectants completely show reduced ice crystal formation.
Freezing Medium and Vials
- The freezing medium consists of basal culture medium combined with serum and cryoprotectant in precise proportions.
- Select freezing vials like polypropylene cryovials that provide strong sealing and low-temperature durability.
Freezing Protocol
- Cooling Rate: Controlling the cooling rate is key, usually at a decrease of 1°C per minute. Too fast or too slow cooling rates can cause cell damage.
- Comparison of Programmed Freezers and Mr. Frosty: Programmed freezers offer precise cooling rate control but are more expensive. Mr. Frosty is a simple cooling device that gradually cools cells in a -80°C freezer and can achieve satisfactory results.
Storage Conditions
- Comparison of Liquid Phase and Vapor Phase Nitrogen Storage: Liquid phase storage offers lower temperatures (-196°C) but poses risks of liquid nitrogen leakage; vapor phase storage is slightly warmer (-150°C or so) but relatively safer.
- Labeling and Inventory Management: Cryovials need to be clearly labeled with cell type, freezing date, etc. Establish an inventory management system with regular stock checks.
Cell Thawing
Thawing Procedure
- Rapid Thawing: Remove the cryovial from liquid nitrogen and immediately place it in a 37°C water bath for rapid thawing, usually completed within 1-2 minutes.
- Immediate Transfer: Transfer the thawed cells immediately into pre-warmed culture medium to dilute the cryoprotectant, reducing its toxicity to the cells.
Initial Post-Thaw Culture
- Centrifugation to Remove Cryoprotectant: Transfer the thawed cell suspension into a centrifuge tube and centrifuge at 1000 rpm for 5 minutes to remove the cryoprotectant in the supernatant.
- Seeding at Appropriate Density: Seed the cells at an appropriate density based on cell type and experimental needs, typically 5×10⁵ to 1×10⁶ cells per square centimeter in culture flasks or dishes.
Monitoring After Recovery
- Observation of Cell Viability and Growth: Examine cell morphology under a microscope; normal cells should resume attachment and growth within 24 hours. Assess cell viability using a cell viability assay kit.
- Key Points for Handling Cells After Initial Recovery: The cells are fragile post-recovery and should be handled carefully within the first 24 hours. Avoid excessive actions such as frequent media changes or passaging.
Common Problems and Solutions
- Low Viability: This may result from inadequate cell viability prior to freezing or improper freezing protocol. Optimizing cell condition before freezing and refining the freezing protocol is necessary.
- Cell Clumping: This may occur due to rapid handling during thawing or excessive centrifugal force. Control the thawing speed and centrifugation parameters.
- Contamination: This might be due to improper sealing of cryovials or contamination during handling. Adhere strictly to aseptic techniques and regularly check for contamination.
Reference
- Murray KA, Gibson MI. Chemical approaches to cryopreservation. Nat Rev Chem. 2022. 6(8):579-593. doi: 10.1038/s41570-022-00407-4. Epub 2022 Jul 18.