From Blur to Clarity: Solving Resolution Limits in Live Cell Imaging
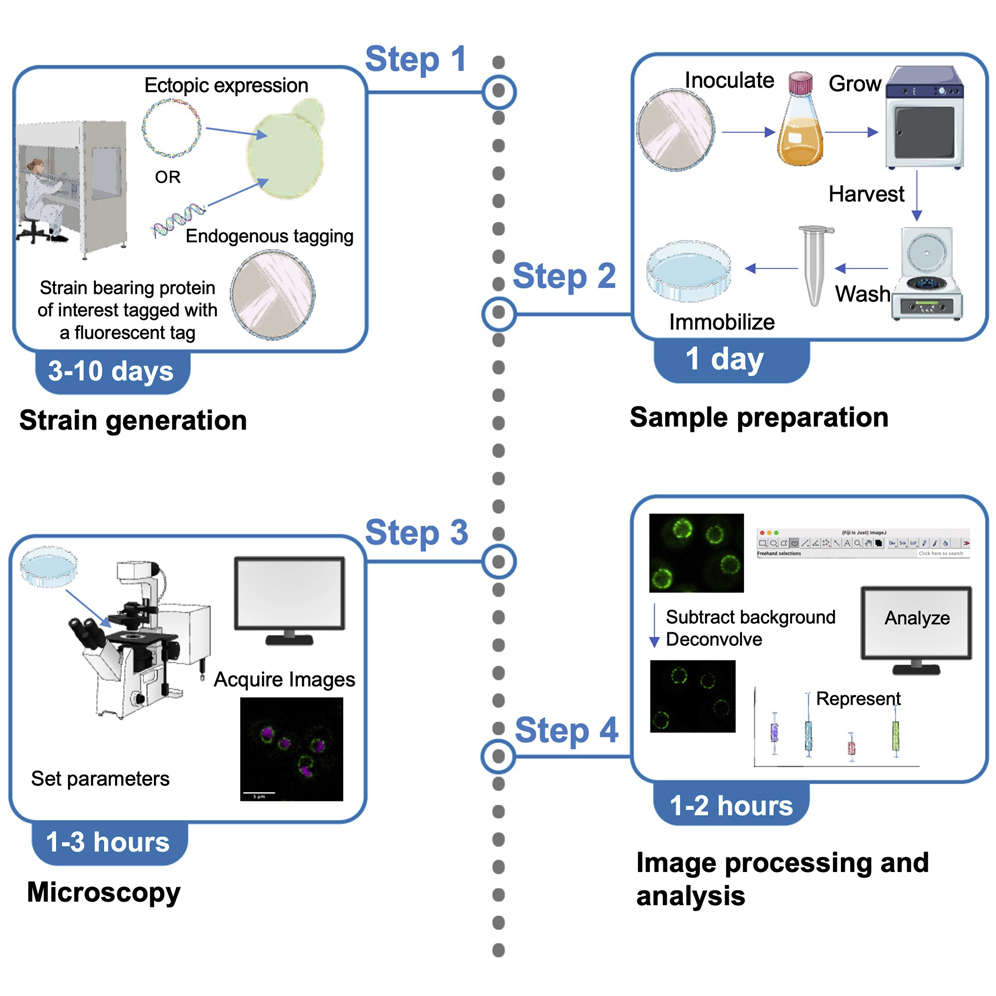 Fig. 1. An adaptable live-cell imaging protocol to analyze organelle morphology in Saccharomyces cerevisiae (Deolal P and Mishra K, 2022).
Fig. 1. An adaptable live-cell imaging protocol to analyze organelle morphology in Saccharomyces cerevisiae (Deolal P and Mishra K, 2022).
Selection of fluorescence labeling tools, optimization of imaging equipment performance, and the accuracy of experimental design and data analysis are three important factors for the successful application of live cell imaging technology. The following sections provide a detailed discussion of these aspects.
Choice of Fluorescent Proteins
Fluorescent proteins are one of the main reagents for live cell imaging technology, and the sensitivity and specificity of experiments depend to a large extent on the selection of fluorescent proteins. Considerations for selecting fluorescent proteins include:
- Spectral Characteristics: Different fluorescent proteins require matching excitation/emission wavelengths with imaging equipment and minimizing spectral overlap such as the cross-excitation problem between GFP and YFP when labeling multiple colors. Common fluorescent proteins include green fluorescent protein (GFP), BFP (blue), and near-infrared fluorescent protein (such as iRFP). Near-infrared fluorescent protein is used for deep tissue imaging as it has a strong ability to penetrate tissue.
- Brightness and Stability: High-brightness fluorescent proteins can help reduce exposure time and avoid phototoxicity. Variants with high photostability (such as Photoactivatable GFP) are preferred for long-term tracking experiments.
- Maturation Time and Toxicity: Fast-maturing fluorescent proteins (such as Superfolder GFP) can reduce the experimental waiting time, while low-toxicity fluorescent proteins (such as mRuby3) can better avoid interference with the physiological activity of cells.
- Functional Extensibility: Photo-switchable fluorescent proteins (such as PA-GFP, Dendra2) can be activated by specific wavelengths of light to achieve spatiotemporal labeling of subcellular structures. Fluorescent protein-based biosensors (such as GCaMP, Cameleon) can be used for real-time monitoring of physiological parameters such as calcium ions and pH.
Imaging Equipment and Technologies
The capabilities of imaging equipment ultimately set the resolution, speed, and depth of live cell imaging. Common devices and methods include:
- Widefield Microscope: A standard microscope for fast and large-field imaging, with relatively low resolution (∼200 nm). Used for observing collective cellular behaviors (e.g., migration).
- Confocal Microscope: Uses pinhole filtering to reject out-of-focus light to achieve optical sectioning (down to 180 nm resolution) and is suitable for 3D structural reconstruction (e.g., organelle morphology).
- Light Sheet Microscope: Illuminates a sample with a thin sheet of light, greatly reducing phototoxicity, and is suitable for long-term live imaging (e.g., dynamics of embryonic development).
- Super-Resolution Microscope: Super-resolution techniques, such as STED (stimulated emission depletion microscopy), PALM, and STORM (photoactivated localization microscopy), overcome the diffraction limit of light (resolution down to 20-50 nm) and are used for observing nanoscale structures (e.g., microtubule dynamics).
- High-Speed Imaging Technology: Combination of sCMOS camera with high-speed control software to achieve millisecond time resolution, used for fast dynamic events (e.g., vesicle fusion and cell division).
Experimental Design and Data Analysis
Experimental design and data analysis are key to ensuring the accuracy and reliability of live cell imaging results:
Experimental design:
- Control Group Design: Set up positive and negative controls (such as the treatment of compounds known to activate signaling pathways, and untreated cells) to exclude non-specific signals.
- Time point selection: Choose imaging intervals based on cellular behavior dynamics including migration speed and signal transduction duration for example every 5 minutes.
- Number of samples and repeats: Ensure that the number of cells in each experimental group is sufficient (usually ≥50) and the experiment is repeated at least three times independently to test the reproducibility of the results.
Data analysis:
- Image preprocessing: Raw images undergo denoising and contrast adjustment along with background signal correction before analysis.
- Image segmentation and feature extraction: Image segmentation is often used to separate cells or cell structures in images. CellProfiler software can automatically identify and segment cells in an image and extract morphological features such as area, perimeter, roundness, etc.
- Analysis of dynamic processes: For live cell imaging data, it is also necessary to analyze the dynamic processes. For example, software like ImageJ can be used to analyze the frequency, distance, time, and velocity of cell membrane protrusions in order to observe the dynamic changes in cellular behaviors.
- Statistical Analysis: Statistical analysis methods are used to evaluate whether the experimental results are statistically significant. Mean, standard deviation, variance analysis, etc. can be used to determine whether there are significant differences between the treatment and control groups.
- Data visualization: Visualization software such as FlowJo and FCS Express can display the data analysis results in graphic form (such as bar charts, line graphs, etc.) to intuitively show the experimental results.
Challenges and Solutions in Live Cell Imaging
Technical challenges
- Phototoxicity and Light-Induced Damage: High-intensity lasers can damage cells, altering their physiological state or even causing cell death. In addition, long-term imaging can exacerbate photobleaching, decreasing the stability of the fluorescent signal.
- Resolution Limitations: Resolution in traditional microscopy is limited by the diffraction limit, which hinders high-resolution imaging of subcellular structures. Super-resolution techniques such as STED, STORM, and PALM can break through the diffraction limit. However, their use in living cells still has problems such as increased phototoxicity and limited imaging speed.
- Cell Focus Drift: During long-term imaging, cells may shift positions due to metabolic activities or environmental changes, leading to focus drift. This not only affects image quality but may also skew experimental results.
Strategies
- Selection of Low-Phototoxicity Fluorescent Proteins: Researchers can select fluorescent proteins that have low-phototoxicity and high-photostability, such as near-infrared fluorescent proteins which are known to have better photostability for long-term imaging.
- Optimization of Imaging Conditions: Optimizing the imaging conditions, such as the laser power, exposure time, and frame rate can reduce light-induced damage. For example, low-power lasers and short exposure times can limit the amount of light-induced cellular damage.
- Adoption of Advanced Imaging Techniques: Advanced imaging techniques, such as multiphoton microscopy, structured illumination microscopy (SIM), and PALM can improve the resolution of images while reducing light-induced damage.
- Employment of Autofocus Systems: Autofocus systems dynamically adjust the microscope's focal plane to maintain image clarity during long-term imaging. Automated imaging systems, such as the Revolution automated imaging system, are often equipped with autofocus features to compensate for focus drift.
- Environmental Control and Cell Culture Optimization: Maintaining strict control over the culture environment, such as temperature, pH, and oxygen levels, is important to keep cells healthy. Optimizing anoxic culture media and using continuous culture techniques can help reduce the accumulation of waste products and improve cell viability.
- Motion Correction Algorithms: Image registration software, such as StackReg or TurboReg, can be used to globally or locally align time-series images. Physically fixing the position of the cells using microfluidic chips can also help reduce physical motion during imaging.
Reference
- Deolal P, Mishra K. An adaptable live-cell imaging protocol to analyze organelle morphology in Saccharomyces cerevisiae. STAR Protoc. 2022. 3(1):101124.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| Cell-based Screening and Profiling Services | Speed up your drug discovery with Creative Bioarray's cell-based screening and profiling services. |