GMP CAR-T & CAR-NK Manufacturing
- Service Details
- Features
- FAQ
- Explore Other Options
The fast progression of cell therapy technologies has brought CAR-T (Chimeric Antigen Receptor T-Cell) and CAR-NK (Chimeric Antigen Receptor Natural Killer Cell) therapies to the forefront as advanced solutions in cancer treatment. By engineering patients' immune cells to more effectively identify and attack cancer cells, these innovative treatments offer new hope for cancer patients.
Our GMP CAR-T & CAR-NK CDMO services provide biopharma innovators with end-to-end manufacturing solutions for next-generation cell therapies. Utilizing extensive expertise in GMP production and regulatory compliance, we offer support for both clinical and commercial-scale manufacturing. From process development to product release, we ensure seamless compliance and operational excellence.
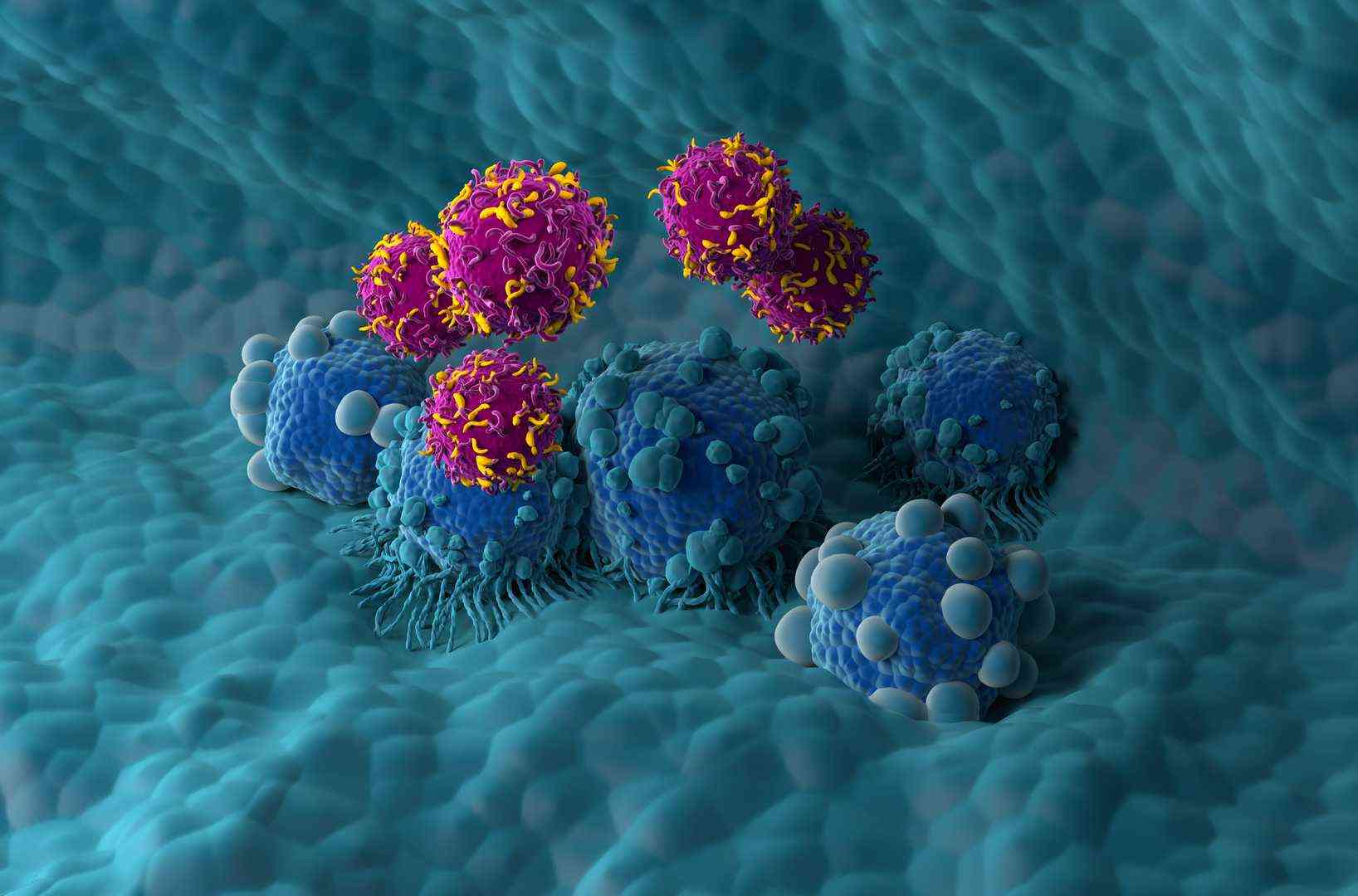
Tailored and Scalable Solutions
We tailor our services to meet the unique requirements of CAR-T and CAR-NK programs, facilitating swift transition from preclinical to clinical stages.
Flexible CAR Delivery Systems
- Viral Vectors: Lentiviral vectors (LVV), retroviral vectors (RVV), and adeno-associated viruses (AAV) for stable CAR integration.
- Non-Viral Modalities: mRNA/LNP and electroporation for transient or permanent CAR expression.
- Gene Editing: TALENs, and base editing for precision genome engineering.
Process Optimization & Customization
- Closed-System Bioprocessing: Transition to fully closed systems to enhance sterility and scalability.
- Media & Reagent Flexibility: Adaptation of xeno-free or defined culture conditions to align with client specifications.
- Automation Integration: Minimize manual intervention with robotic liquid handling and bioreactor controls.
Rigorous Quality Control & Testing
- Identity & Physical / Chemical properties
- Purity: Tests include BSA, E1A, SV40, RetroNectin, magnetic beads, and bovine serum albumin residuals.
- Strength: Assessments include cell viability, CAR transduction efficiency, cell killing assays, and CAR copy number.
- Safety: Including mycoplasma testing, endotoxin detection, sterility testing and replication competent virus testing.
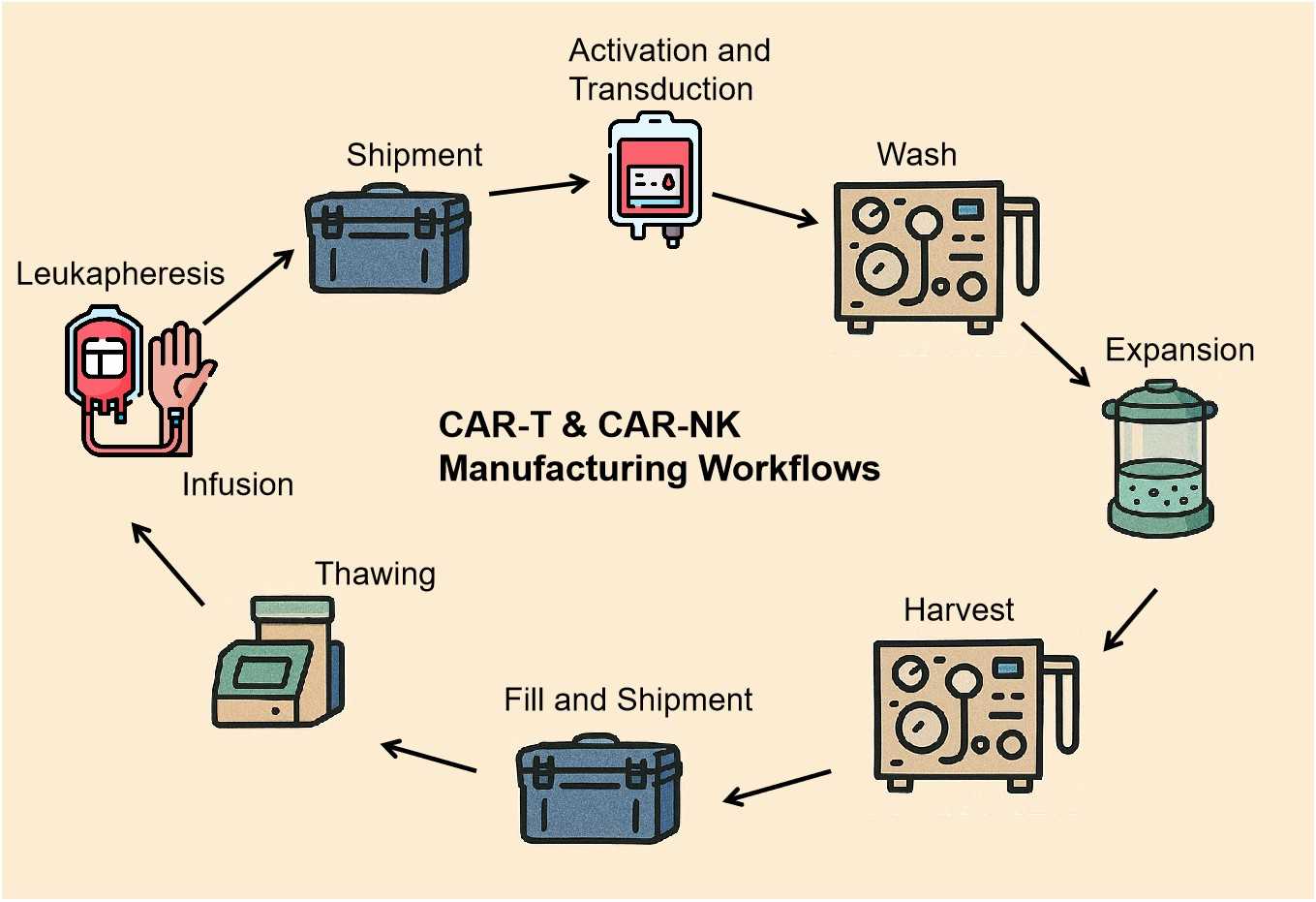
Workflow
Our validated workflow ensures product integrity, potency, and safety at every stage:
1
Quality assessment of leukapheresis products (viability, cell count, sterility).
2
Magnetic or FACS-based enrichment and activation via CD3/CD28 beads or cytokines (IL-2, IL-15).
3
Lentiviral transduction or mRNA/LNP electroporation for CAR delivery, incorporating specific receptor knockout strategies to enhance efficacy.

4
Cell culture in bioreactors or G-Rex flasks to achieve therapeutic doses. Final formulation in cryoprotective media (e.g., DMSO + human serum albumin).
5
Sterility, potency, and identity testing prior to patient administration.
Why Partner with Us?

Proven Track Record
Extensive experience in filing numerous INDs for CAR cell therapies, streamlining client regulatory pathways globally.
Cost Efficiency
In-house GMP plasmid and viral vector production. Closed-system automation reducing manual labor, contamination risks, and batch failures.

Scientific Leadership
Collaborate with our R&D team to co-develop novel CAR constructs or manufacturing innovations.
FAQ
1. Why is GMP-grade CAR-T and CAR-NK manufacturing necessary?
GMP ensures drug production quality management standards, ensuring safety, efficacy, and consistency for biologics like CAR-T and CAR-NK therapies used in humans.
2. Does your CDMO service support IND applications?
Yes, our services fully support IND preparation and submission, offering comprehensive solutions from process development to quality control.
3. Is large-scale production supported by your platform?
Yes, we support both small and large-scale production with advanced equipment and flexible processes tailored to your needs.
4. What is the typical production timeline?
Timelines vary by project, with development to delivery potentially as short as two weeks, depending on process complexity and scale.
Explore Other Options