Understanding Immunogenicity Assays: A Comprehensive Guide
What is Immunogenicity?
Immunogenicity is the ability of a biological product to induce an immune response, resulting in the formation of neutralizing and non-neutralizing antibodies in the body. These responses can lead to undesirable effects in patients, such as allergic reactions, increased drug toxicity, and decreased drug efficacy. Therefore, immunogenicity assessment is an important step in biological product development to ensure safety and efficacy.
Cell-Based Assays for Immunogenicity Evaluation
Cell-Based Assays are one of the critical tools for immunogenicity evaluation. These methods evaluate immune responses through the detection of the activation, proliferation, or secretion function of specific cell populations. They are highly sensitive and specific, allowing the data collected to be closely related to biological function and help to predict and manage potential immunogenic risks. Some commonly used methods include:
T cell proliferation assay:
Evaluation of immunogenicity by measuring T cell proliferation on specific antigen stimulation. It is used for the evaluation of immunogenicity of biological agents (such as monoclonal antibodies) and immunogenic effects of vaccines.
(1) Peripheral Blood Mononuclear Cells (PBMC) Proliferation Assay: PBMCs are the most commonly used cell type for T cell proliferation assay. Through the co-culture of PBMCs with antigens or peptide pools, the proliferation of CD4+ and CD8+ T cells can be detected. It is applicable for the evaluation of immunogenicity of vaccines or antibody therapeutics.
(2) Dendritic Cells (DCs) - T Cell Co-culture Assay: Through the co-culture of antigen-presenting cells (such as DCs) with T cells, it simulates the antigen presentation in vivo and has a better detection of T cell immune response. However, it requires a large number of DCs and complicated steps, so it is limited in the application.
(3) DCs-T Cell Assay: The simplified method of DCs-T cell co-culture assay. DCs extracted from peripheral blood mononuclear cells show antigen-specific T cell proliferation when cultured together with T cells. It has a lower sensitivity than the DCs-T cell co-culture assay but is easy to operate and is suitable for preliminary screening.
Advantages:
- A direct evaluation of the T cell immune memory level and antigen-specific response.
- Combined with other immunological indicators (such as cytokine secretion) to make a comprehensive evaluation, which can increase the comprehensive detection and accuracy.
Disadvantages:
- For low affinity or short half-life antigen epitopes, the T cell proliferation response may be weak and difficult to detect.
- Flow cytometry requires high-quality samples and standard operation, otherwise it will affect the accuracy of results.
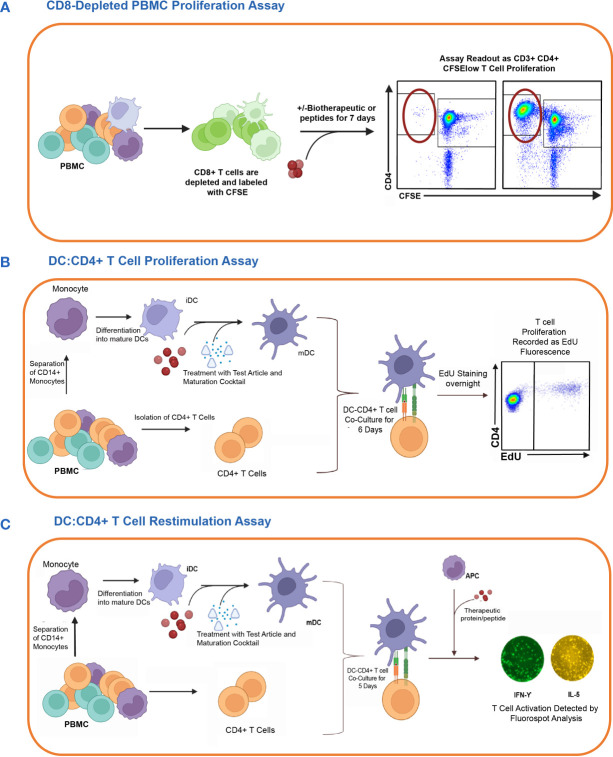 Fig. 1. In vitro cell-based assay methods used to detect CD4 T cell responses to therapeutic proteins in healthy donors. The figure shows the schematic representation of three different T cell assay formats used to assess the risk of raising a CD4 T cell response (Walsh RE, Nix A, et al., 2024).
Fig. 1. In vitro cell-based assay methods used to detect CD4 T cell responses to therapeutic proteins in healthy donors. The figure shows the schematic representation of three different T cell assay formats used to assess the risk of raising a CD4 T cell response (Walsh RE, Nix A, et al., 2024).
Cytokine release assay:
Detect the immunogenicity of an antigen through cytokine release upon specific antigen stimulation. Used to evaluate the immunogenicity of biologics, vaccines, and other protein therapeutics.
Method: Incubate PBMCs or certain immune cells (e.g., dendritic cells) with the antigen to be tested, and detect cytokine levels (e.g., IFN-γ, IL-2, TNF-α) via ELISA, MSD, or other cytokine detection techniques.
Advantages:
- Speed: Many cytokine release assays can be performed within a short period. For example, changes in IL-2 secretion can be detected within three days.
- High sensitivity: Low concentrations of cytokines can be measured with high precision by flow cytometry or electrochemiluminescence immunoassays such as ELISPOT.
Drawbacks:
- Low specificity: Some cytokines may be induced by non-specific stimuli. Therefore, it is better to use other techniques (e.g., ELISPOT) in combination.
- Dependent on Experimental Conditions: The accuracy of results may be affected by sample handling, antigen selection, and culture conditions.
Mixed lymphocyte reaction (MLR):
Measuring immunogenicity by detecting interactions between different lymphocytes. Primarily for organ transplant matching and assessing immune rejection risk.
Method: Mixed donor and recipient PBMCs at a specific ratio, typically using HLA-mismatched cells as stimulants to mimic allogeneic immune response. T cell proliferation and activation are detected using flow cytometry, single-cell RNA sequencing, or IFN-γ secretion. MLR is further divided into unidirectional and bidirectional types:
(1) Unidirectional MLR: Donor lymphocytes serve as stimulants while recipient lymphocytes act as responders and are used to measure donor cell immunogenicity.
(2) Bidirectional MLR: Interactions between donor and recipient lymphocytes are measured to assess immune compatibility.
Advantages:
- Simulates real immune responses: MLR provides a tool to measure the immunotherapy or transplant rejection risk by mimicking allogeneic immune response.
- High specificity: MLR responses are antigen-specific and require no pre-sensitization to donor antigens.
- Wide application: Suitable for measuring the immunogenicity of different cell types (mesenchymal stem cells, embryonic stem cells).
Disadvantages:
- Long duration: MLR tests usually take several days, and results vary depending on the type of donor cells.
- Donor variation effect: Genetic variation among donors can lead to varying results.
- High technical requirements: Careful control of experimental conditions such as cell ratios and culture time is necessary for reliable results.
Cytotoxicity assay:
Measuring immunogenicity by detecting the activity of cytotoxic T lymphocytes (CTLs) upon antigen stimulation. Used for measuring immunogenicity of tumor vaccines and immunotherapies.
Method: Culturing PBMCs with the test antigen and detecting CTLs' killing ability towards target cells using flow cytometry or lactate dehydrogenase (LDH) release assay.
Reference
- Walsh RE, Nix A, et al. Preclinical immunogenicity risk assessment of biotherapeutics using CD4 T cell assays. Front Immunol. 2024. 15:1406040.