iPSC Differentiation
- Service Details
- Workflow
- Features
- Explore Other Options
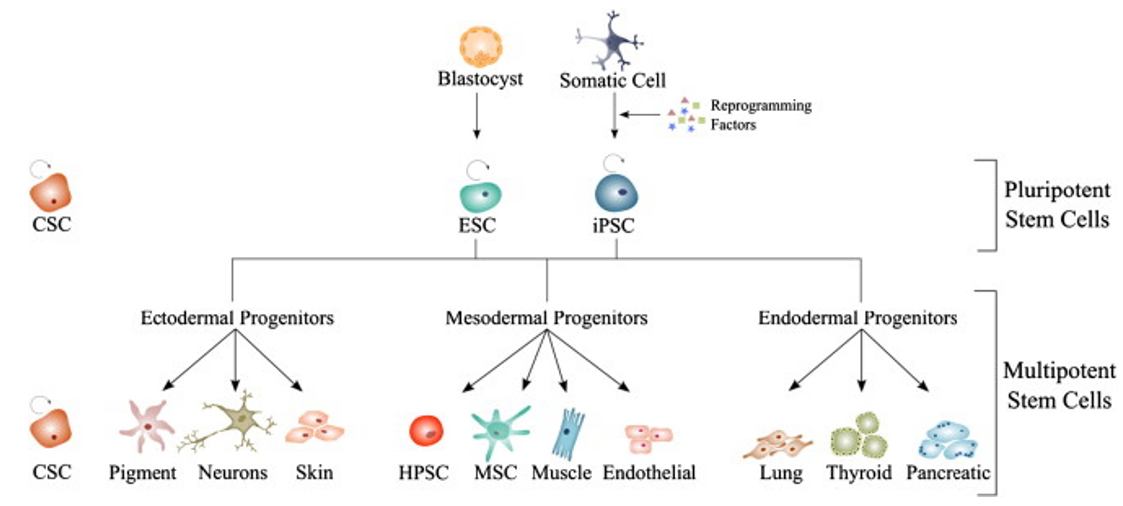
Differentiation is the process where an unspecialized cell acquires cellular traits that allow it to perform specialized functions. Indeed, the actual potential of iPSCs rest in their direct differentiation to various types of somatic cells. In order to use iPSCs to treat disease or as research tools to study diseases, fully functioning specialized cells are required for testing potential drugs or transplanting healthy cells in clinical stages. Creative Bioarray has developed protocols for iPSC differentiation to various lineage-committed cell types thereby expanding the scope of your research, drug discovery, or screening projects.
Advantages of iPSC-Derived Cellular Models
- Accurate Simulation: These models retain human genetic backgrounds and closely mimic organ physiology and pathology, providing reliable data.
- Precision Disease Models: Created from patient cells, they accurately reflect disease mechanisms, aiding research and drug screening.
- Personalized Medicine Support: Aligning with individual genetics, they help predict drug efficacy and safety, promoting personalized treatments.
- Scalability and Consistency: iPSCs can be robustly expanded, with standardized processes ensuring consistent quality for large-scale studies.
- Ethical Advantage: Derived from adult cells, they avoid ethical issues, enhancing acceptance in stem cell research and applications.
Our iPSC Differentiation Services
- Provide differentiation services from both healthy and diseased iPSC lines to meet diverse modeling needs.
- Develop co-culture and organoid models across multiple lineages, closely simulating physiological and pathological environments of human organs.
- Ensure high purity and efficient output, with optimal cell viability and functionality.
- Conduct comprehensive functional validation to ensure the complete functionality of the cells.
iPSC Differentiation Service Categories
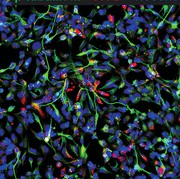
The differentiation of iPSCs to neurons and related cell types is crucial for neurobiology and disease research, especially given the limited availability of clinically relevant in vitro models. Creative Bioarray specializes in directing hiPSCs into diverse neuronal subtypes, including: Neural stem cells (NSCs), Neural Progenitor cells (NPCs), Mature Astrocytes, Dopaminergic neurons, Motor neurons progenitor, Cerebral cortical neurons, Oligodendrocytes, Microglia. Glutamatergic neurons, Cholinergic neurons, Purkinje cells, Neural crest cells, Astrocytes
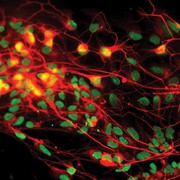
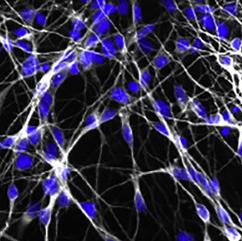
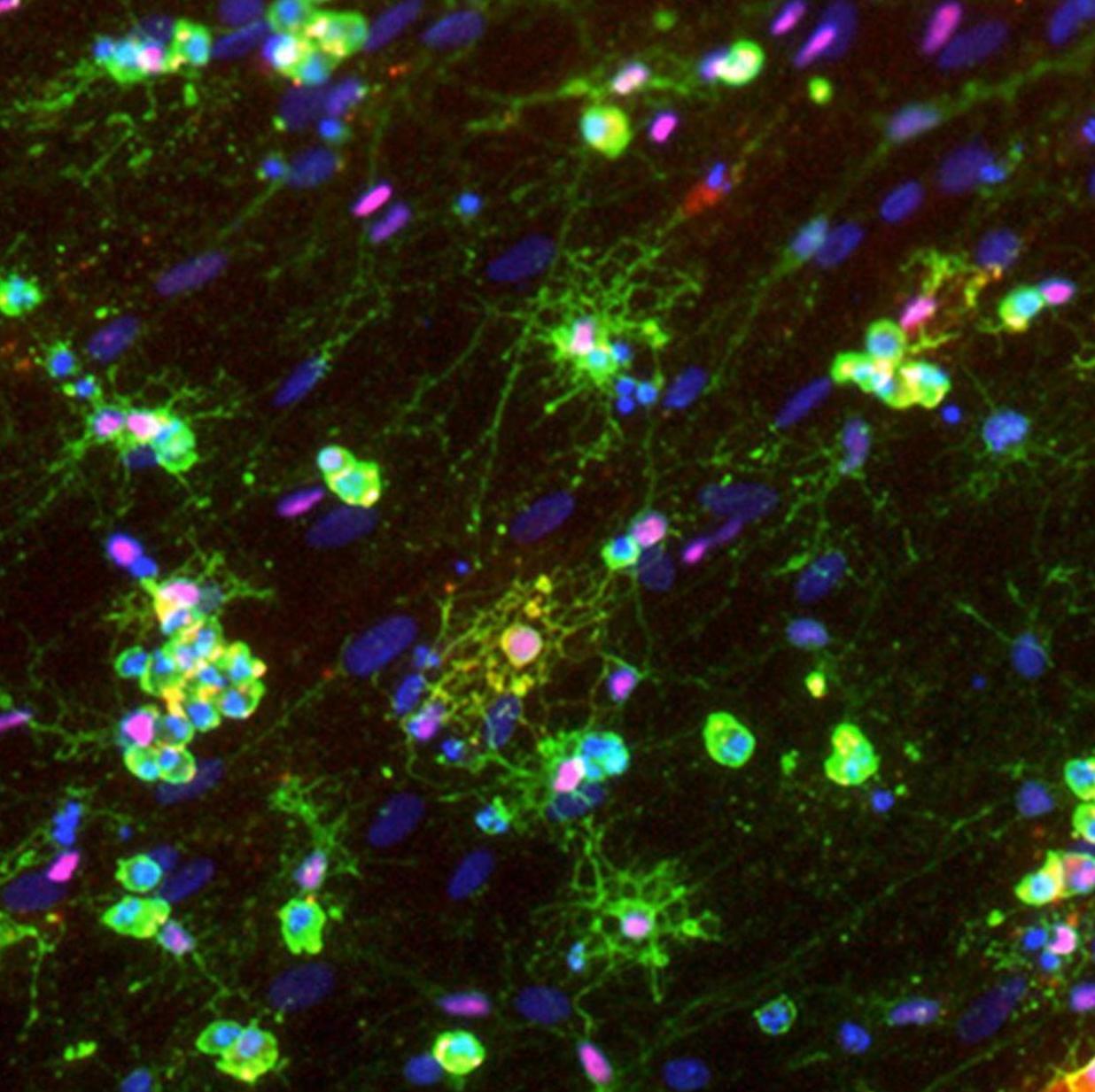
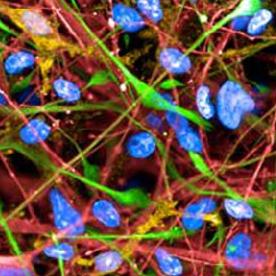
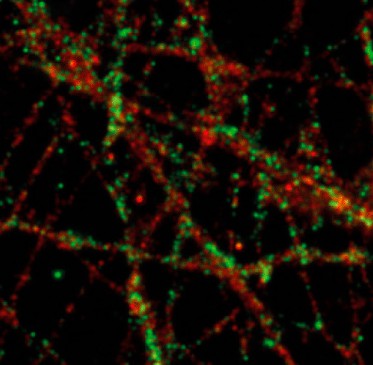
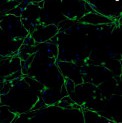
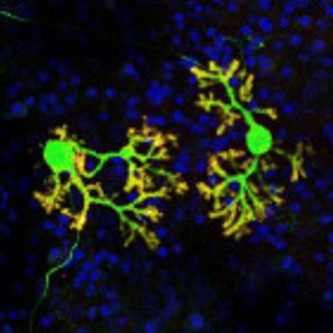
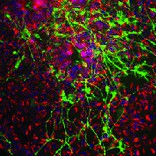
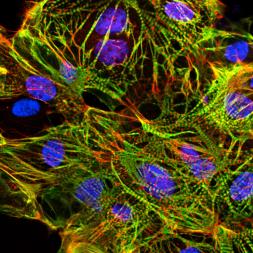
Creative Bioarray offers ready-to-use and functional cardiomyocytes that are desirable in vitro models for high content toxicity and drug screening, and cell regeneration research. We provide iPSC-derived cardiomyocytes from healthy or diseased patients.
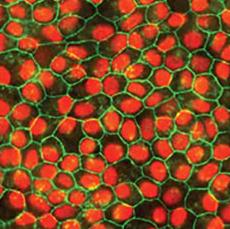
Human iPSC-derived retinal pigment epithelium (RPE) provides a physiologically functional cell model to study ocular diseases. Creative Bioarray provides high quality RPE with typical cobblestone morphology and pigmentation, and high purity RPE expressing RPE-specific markers BEST1/rpe65.
Creative Bioarray generates iPSC-derived immune cells including CD34+ progenitor cells, CD8+T cells, NK cells, monocytes, and dendritic cells from clonal master iPSC line(s) using highly optimized and efficient differentiation protocols for preclinical applications.
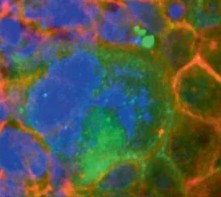
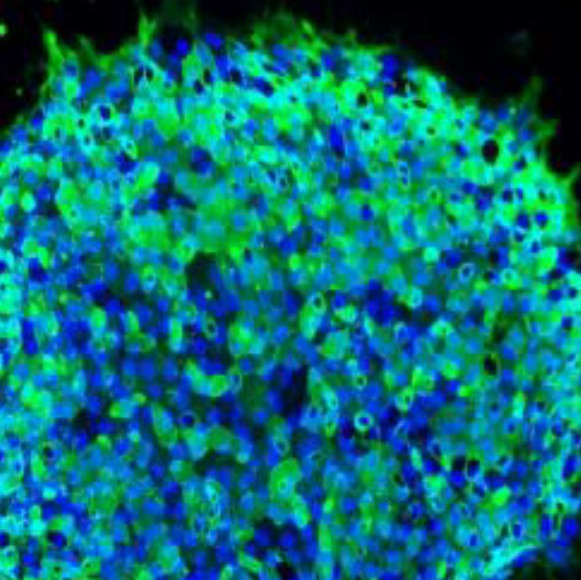
iPSC-derived hepatocytes as developing liver disease models, are feasible and physiologically relevant alternative for discovering therapeutic treatments, drug targets, and understanding drug induced liver inquiries (DILI). Creative Bioarray's hepatocyte differentiation services include: expansion of iPSCs, progenitor cells differentiation and characterization, terminal differentiation, and characterization of differentiated cells by ICC (per markers).
Workflow
Step 1: Initial Consultation
Understand the client’s research objectives and specific requirements.
Step 3: Quotation
Provide a detailed and structured pricing model.
Step 5: Deliverable Handoff
Deliver the final results to the client.

Step 2: Customized Planning
Our professional research team designs a tailored experimental plan for the client.
Step 4: Finalization and Contract Signing
Execute the project in strict accordance with predetermined milestones to ensure control over the project timeline.
Features

Reliable, High-Quality Results
By using cutting-edge technology and standardized procedures, we ensure reliable and high-quality differentiation, allowing researchers to focus on discoveries.

Scalability and Consistency
Our optimized differentiation protocols guarantee consistent and reproducible results, suitable for both small and large-scale research.

Fast Turnaround, Customized Solutions
We prioritize efficiency to quickly meet client needs and offer customizable solutions to fully accommodate specific research requirements.
If you have any special need in iPSC differentiation service, do not hesitate to contact us for this special service. Please let us know what you need and we will accommodate you. We are looking forward to working with you in the future.
References
- Kaebisch C, Schipper D, et al. The role of purinergic receptors in stem cell differentiation. Comput Struct Biotechnol J. 2014. 13:75-84.
- Pietrucha K, Zychowicz M, et al. Functional properties of different collagen scaffolds to create a biomimetic niche for neurally committed human induced pluripotent stem cells (iPSC). Folia Neuropathol. 2017. 55(2):110-123.
- Akkouh IA, Ueland T, et al. Decreased IL-1β-induced CCL20 response in human iPSC-astrocytes in schizophrenia: Potential attenuating effects on recruitment of regulatory T cells. Brain Behav Immun. 2020. 87:634-644.
- Han C, Chaineau M, et al. Open Science Meets Stem Cells: A New Drug Discovery Approach for Neurodegenerative Disorders. Front Neurosci. 2018. 12:47.
- Hedegaard A, Monzón-Sandoval J, et al. Pro-maturational Effects of Human iPSC-Derived Cortical Astrocytes upon iPSC-Derived Cortical Neurons. Stem Cell Reports. 2020. 15(1):38-51.
- Plastini MJ, Desu HL, et al. Transcriptional abnormalities in induced pluripotent stem cell-derived oligodendrocytes of individuals with primary progressive multiple sclerosis. Front Cell Neurosci. 2022. 16:972144.
- Bachmann S, Linde J, et al. DNA Methyltransferase 1 (DNMT1) Shapes Neuronal Activity of Human iPSC-Derived Glutamatergic Cortical Neurons. International Journal of Molecular Sciences. 2021. 22(4):2034.
- Duan L, Bhattacharyya BJ, et al. Stem cell derived basal forebrain cholinergic neurons from Alzheimer's disease patients are more susceptible to cell death. Mol Neurodegener. 2014. 9:3.
- Morino H, Matsuda Y, et al. A mutation in the low voltage-gated calcium channel CACNA1G alters the physiological properties of the channel, causing spinocerebellar ataxia. Mol Brain. 2015. 8:89.
- Shi L, Huang L, et al. Modeling the Pathogenesis of Charcot-Marie-Tooth Disease Type 1A Using Patient-Specific iPSCs. Stem Cell Reports. 2018. 10(1):120-133.
- Lendemeijer B, Unkel M, et al. Human Pluripotent Stem Cell-Derived Astrocyte Functionality Compares Favorably with Primary Rat Astrocytes. eNeuro. 2024. 11(9):ENEURO.0148-24.2024.
- Reich M, Paris I, et al. Alzheimer's Risk Gene TREM2 Determines Functional Properties of New Type of Human iPSC-Derived Microglia. Front Immunol. 2021. 11:617860.
- Markert EK, Klein H, et al. Transcriptional comparison of adult human primary Retinal Pigment Epithelium, human pluripotent stem cell-derived Retinal Pigment Epithelium, and ARPE19 cells. Front Cell Dev Biol. 2022. 10:910040.
- Riegler J, Ebert A, et al. Comparison of Magnetic Resonance Imaging and Serum Biomarkers for Detection of Human Pluripotent Stem Cell-Derived Teratomas. Stem Cell Reports. 2016. 6(2):176-87.
- A tissue-engineered artificial human thymus from human iPSCs to study T cell immunity. Nat Methods. 2022. 19(10):1191-1192.
- Klaihmon P, Kang X, et al. Generation and Functional Characterization of Anti-CD19 Chimeric Antigen Receptor-Natural Killer Cells from Human Induced Pluripotent Stem Cells. International Journal of Molecular Sciences. 2023. 24(13):10508.
- Wang XM, Yik WY, et al. Induced pluripotent stem cell models of Zellweger spectrum disorder show impaired peroxisome assembly and cell type-specific lipid abnormalities. Stem Cell Res Ther. 2015. 6:158.
Explore Other Options