Type II Collagenase-Induced Osteoarthritis (OA) Model
Creative Bioarray possesses the requisite expertise and extensive pharmaceutical industry experience to assist our clients in assessing the therapeutic potential of test agents for the treatment of osteoarthritis (OA). We have successfully developed a highly stable OA model, induced by type II collagenase. Our model is designed to accurately simulate the pathophysiological conditions of OA, enabling precise evaluation of the efficacy and safety of novel therapeutic candidates.
OA is the most prevalent degenerative joint disorder, affecting critical joint components such as articular cartilage, synovium, and subchondral bone. A comprehensive understanding of OA's pathophysiological mechanisms is vital for the advancement of more effective treatments. Collagenase plays a significant role in the induction of knee osteoarthritis, primarily targeting joint structures like tendons and ligaments, which are rich in collagen type 1. This enzyme's action leads to joint instability, with minimal direct impact on joint cartilage. The injection of collagenase into the joint cavity serves as a method to experimentally induce OA, offering valuable insights into the disease's progression and potential therapeutic targets.
Our Type II Collagenase-Induced Osteoarthritis (OA) Model
- Available Animal
Rat
- Modeling Method
After anesthetization, rats are given the intra-articular injection of type II collagenase on day 1 and day 4.
- Endpoints
- Behavioral test: open filed test
- Histology analysis: H&E staining
- Mankin score
- Other customized endpoints
Example Data
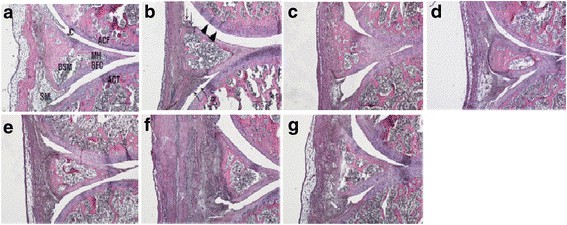 Fig. 1 Histopathological changes (H&E stain) in the knee joint after ChondroT administration in collagenase induced arthritis rats. a Intact, b Control, c Indomethacin, d Joins Tab, e ChondroT50, f ChondroT100, g ChondroT200. (Jeong et al. 2018)
Fig. 1 Histopathological changes (H&E stain) in the knee joint after ChondroT administration in collagenase induced arthritis rats. a Intact, b Control, c Indomethacin, d Joins Tab, e ChondroT50, f ChondroT100, g ChondroT200. (Jeong et al. 2018)
In addition, we also provide other OA models that maybe you are interested in:
- Monosodium Iodoacetate (MIA)-Induced Osteoarthritis (OA) Model
- Papain-Induced Osteoarthritis (OA) Model
- Medial Meniscal Tear (MMT)-Induced Osteoarthritis (OA) Model
- Anterior Cruciate Ligament Transection (ACLT)-Induced Osteoarthritis (OA) Model
Quotation and Ordering
Creative Bioarray is committed to enhancing our in vivo pharmacology capabilities. We are dedicated to meeting the unique requirements of our clients by offering customized models tailored to their specific compound or discovery program. If you are interested in our services, please feel free to contact us at any time or submit an inquiry to us directly.
Reference
- Jeong, J., et al. Anti-osteoarthritic effects of ChondroT in a rat model of collagenase-induced osteoarthritis. BMC complementary and alternative medicine, 2018, 18: 1-10.
For research use only. Not for any other purpose.
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Oral Mucositis Model
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
- Graft-versus-host Disease (GvHD) Models
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Schizophrenia Model
- Depression Models
- Pain Models
-
Metabolic Disease Models
- Type 1 Diabetes Mellitus Model
- Type 2 Diabetes Mellitus Model
- Animal Model of Hyperuricemia
-
Nonalcoholic Fatty Liver Disease Model
- High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Methionine and Choline Deficient (MCD) Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Gubra-Amylin NASH (GAN) Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Streptozotocin (STZ) Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- High Fat Diet-Induced Obesity Model
- Diabetic Foot Ulcer (DFU) Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthotopic Kidney Transplantation Model
- Benign Prostatic Hyperplasia (BPH) Model
- Peritoneal Fibrosis Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
- Otology Disease Models