Unraveling the Role of hERG Channels in Drug Safety
In the realm of drug discovery and development, the assessment of hERG (human Ether-à-go-go-Related Gene) channel toxicity plays a vital role. The hERG channels, which are potassium ion channels, are of particular interest due to their involvement in cardiac repolarization. Adverse effects on the QT interval, resulting from hERG channel dysfunction, can lead to severe cardiotoxicity.
Structure and Function of hERG Channels
The hERG channels are integral membrane proteins comprised of four subunits, each containing six transmembrane domains. These subunits assemble to form functional tetrameric channels in the cell membrane. The hERG channel subunits are encoded by the hERG gene (KCNH2) and are widely expressed in cardiac tissues, as well as in other organs such as the brain, kidneys, and smooth muscle cells.
The primary function of hERG channels is contributing to the repolarization phase of the cardiac action potential. Upon depolarization, hERG channels open, allowing the outflow of potassium ions, leading to the cardiac myocytes' repolarization. This repolarization phase is crucial for the proper rhythm and function of the heart.
Role of hERG Channels in Cardiac Electrophysiology
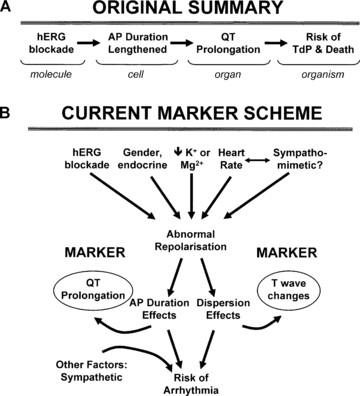 Fig. 1 The relationship between hERG blockade and arrhythmogenic risk. (Witchel HJ, 2011)
Fig. 1 The relationship between hERG blockade and arrhythmogenic risk. (Witchel HJ, 2011)
The role of hERG channels in cardiac electrophysiology is essential for maintaining the heart's normal function. Dysfunction or inhibition of hERG channels can result in delayed repolarization, leading to prolonged action potentials and an increased risk of arrhythmias. Specifically, the inhibition of hERG channels can cause QT interval prolongation on the electrocardiogram (ECG).
Prolongation of the QT interval is associated with an increased risk of developing life-threatening ventricular arrhythmias, such as Torsades de Pointes (TdP). TdP is characterized by a rapid, irregular heart rhythm that can degenerate into ventricular fibrillation, a potentially fatal condition. Therefore, identifying drugs that have the potential to inhibit hERG channels and cause QT interval prolongation is of paramount importance in drug safety assessment.
hERG Toxicity Assessment Methods
Various methods are employed to assess hERG channel toxicity and evaluate the potential for QT interval prolongation. These methods aim to identify drug candidates that have a high risk of hERG channel inhibition and provide valuable insights into their cardiac safety profiles. Some commonly used hERG toxicity assessment methods include:
- Patch-clamp technique. This gold standard method enables the direct measurement of ion channel currents and the determination of drug effects on hERG channel activity. Patch-clamp recordings can accurately assess drug binding kinetics, voltage dependence, and selectivity of hERG channel blockage.
- Automated electrophysiology systems. High-throughput screening platforms that utilize automated patch-clamp technology allow for the efficient evaluation of large compound libraries, enabling the identification of hERG channel inhibitors.
- Expression of hERG channels in non-cardiac cells. Heterologous expression systems, such as cell lines transfected with hERG channel genes, allow for controlled studies of drug interactions with hERG channels. These systems provide insights into the pharmacological properties of compounds and their impact on hERG channel activity.
Regulatory Guidelines and Safety Pharmacology
Regulatory agencies, including the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the U.S. Food and Drug Administration (FDA), have established guidelines and recommendations for assessing hERG channel-related cardiotoxicity during drug development. The primary aim is to ensure patient safety and reduce the risks associated with QT interval prolongation and arrhythmias.
The ICH S7B guideline outlines the requirements for evaluating the potential proarrhythmic risk of drugs by assessing their effects on cardiac repolarization, including hERG channel inhibition. These guidelines emphasize the importance of integrating hERG data with other safety pharmacology endpoints and considering multiple factors in the overall cardiac risk assessment.
Safety pharmacology studies encompass a comprehensive evaluation of a drug candidate's effects on various physiological systems, including the cardiovascular system. In addition to hERG channel assessment, these studies may include evaluations of other ion channels, action potentials, and cardiac contractility. By considering multiple safety parameters, safety pharmacology studies provide a holistic understanding of a drug candidate's potential cardiac risks, enabling informed decision-making during the drug development process.
Creative Bioarray Relevant Recommendations
| Product/Service Types | Description |
| Drug Toxicity Services | Creative Bioarray specializes in identifying potential drug toxicity to different tissues and organs using a panel of in vitro cell-based assays and is an ideal partner for testing the potential toxicities of candidate drug compounds. |
| In Vitro Cardiotoxicity | Our cardiotoxicity testing services include electrophysiology and 3D cell-based cardiotoxicity models. |
| hERG Safety Assay | Creative Bioarray offers an hERG safety assay by the ICH S7B guideline to evaluate compound cardiovascular safety and to support drug development. |
| QT Prolongation Assay | Creative Bioarray offers in vitro QT prolongation using human cardiomyocytes to evaluate the cardiotoxicity of compounds with QT interval as the indicator. |
Reference
- Witchel HJ. (2011). "Drug-induced hERG block and long QT syndrome." Cardiovasc Ther. 29 (4), 251-9.