Diabetic Foot Ulcer (DFU) Model
At Creative Bioarray, we offer comprehensive preclinical pharmacodynamic evaluation services using a well-established Diabetic Foot Ulcer (DFU) model in rodents. This model closely mimics the impaired wound healing observed in diabetic patients and is widely used for the efficacy assessment of therapeutic candidates targeting chronic wounds.
Creative Bioarray's Diabetic Foot Ulcer (DFU) Model
Available Animal
- Rat
- Mouse
Modeling Method
The Diabetic Foot Ulcer (DFU) Model is established by intraperitoneal injection of streptozotocin (STZ) to induce persistent hyperglycemia, followed by the creation of a full-thickness skin excision to mimic wound formation under diabetic conditions.
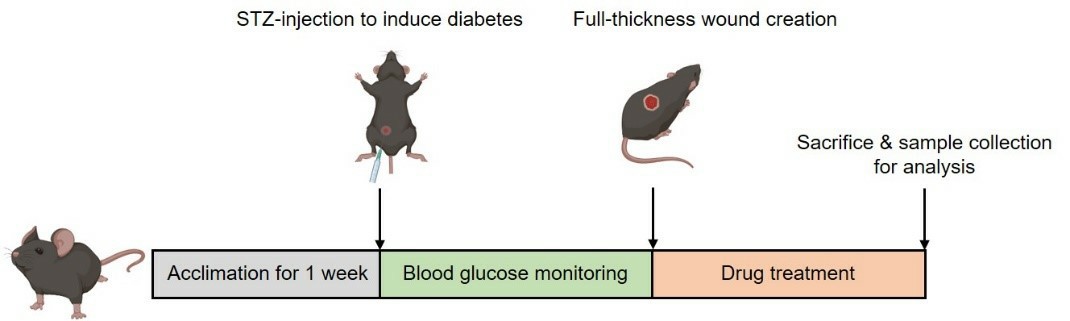 Fig. 1: Schematic diagram of the modeling method of DFU model
Fig. 1: Schematic diagram of the modeling method of DFU model
Evaluation Endpoints
- Blood glucose monitoring (to confirm diabetic state)
- Representative wound images (image-based tracking of healing process)
- Wound area measurement
- Histology analysis (H&E, Masson, IHC)
- Gene and protein expression (qPCR, Western blot)
- Cytokine analysis
- Other customized endpoints according to your specific needs
Typical Study Design (Fully customizable)
| Parameter | Description |
| Model Induction | STZ injection → hyperglycemia confirmation → skin excision |
| Treatment Period | Typically, 1-2 weeks post-wounding |
| Group Setting | Sham, Model, three dose groups of test compound |
| Group Size | n=6-12 per group (adjustable as needed) |
| Delivery Route | Topical, oral, or systemic administration |
| Endpoints | Wound monitoring, blood glucose, histology analysis, etc. |
Deliverables
- Full experimental report (PDF)
- Raw and processed data (e.g., wound images, histology, qPCR, etc.)
- Statistical analysis and interpretation
Key Advantages
- Clinically relevant model: Mimics the impaired healing seen in diabetic patients
- Reproducible & scalable: Ideal for screening and candidate optimization
- Multiple administration routes: topical, oral, or injection, etc.
- Flexible study timelines: Tailored to your compound's mechanism and goals
Example Data
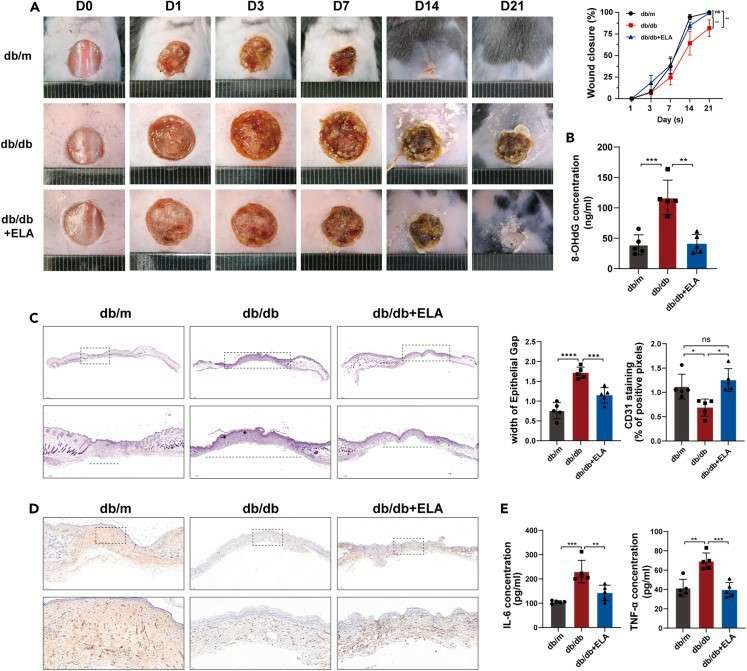 Fig.2: Administration of Elabela in the peri-wound area promotes wound healing in mice with diabetes. (Hong et al., 2023)
Fig.2: Administration of Elabela in the peri-wound area promotes wound healing in mice with diabetes. (Hong et al., 2023)
Quotation and Ordering
Our scientific team is ready to support your wound healing drug development with high-quality, cost-effective in vivo pharmacodynamic data. We offer flexible study options and fast turnaround times tailored to your specific needs.
Contact us today to discuss your project or request a custom proposal and quotation.
Reference
- Hong, Y., et al. Elabela inhibits TRAF1/NF-κB induced oxidative DNA damage to promote diabetic foot ulcer wound healing. iScience. 2023;26(9):107601.
For research use only. Not for any other purpose.
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Oral Mucositis Model
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
- Graft-versus-host Disease (GvHD) Models
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Schizophrenia Model
- Depression Models
- Pain Models
-
Metabolic Disease Models
- Type 1 Diabetes Mellitus Model
- Type 2 Diabetes Mellitus Model
- Animal Model of Hyperuricemia
-
Nonalcoholic Fatty Liver Disease Model
- High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Methionine and Choline Deficient (MCD) Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Gubra-Amylin NASH (GAN) Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Streptozotocin (STZ) Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- High Fat Diet-Induced Obesity Model
- Diabetic Foot Ulcer (DFU) Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthotopic Kidney Transplantation Model
- Benign Prostatic Hyperplasia (BPH) Model
- Peritoneal Fibrosis Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
- Otology Disease Models