Types of Cell Therapy for Cancer
At present, the clinical treatment of tumors is still focused on surgery, radiotherapy, chemotherapy, and other traditional methods, and is gradually being combined with targeted therapy, immunotherapy and other new methods. These methods have achieved better results in the early stage of many tumors and some late-stage treatments, but are still limited by a series of problems such as poor efficacy in late-stage tumors, drug resistance, high heterogeneity of tumors, and damage of normal tissue by treatment, and the heterogeneity and complexity of the tumor microenvironment, as well as differences among individuals also bring challenges to the precision treatment.
Cell therapy applies live cells which have undergone screening, modification, or expansion processes for medical treatment applications. It can repair, replace, or enhance the body's function by the specific biological function of the cell itself. Cell therapy used in cancer treatment consists mainly of adoptive cell transfer approaches like CAR-T and TCR-T cell therapy in addition to tumor infiltrating lymphocytes (TILs) therapy and dendritic cell vaccine therapy. Adoptive cell therapies target tumor-specific antigens to achieve precise tumor killing while dendritic cell vaccines activate immune responses against tumors and provide innovative treatment options for refractory or recurrent cancers.
Immune Cell Therapy
CAR-T cell therapy
CAR-T cell therapy is one of the most popular immune cell therapies currently. It modifies the patient's T cells through genetic engineering technology to express specific chimeric antigen receptors (CARs) on their surface, thereby enabling more effective identification and attack of tumor cells.
Application: CAR-T therapy has made significant progress in the treatment of blood cancers and has been FDA approved for the treatment of certain types of acute lymphoblastic leukemia and non-Hodgkin lymphoma. For instance, the US FDA has approved Kymriah developed by Novartis and Yescarta developed by Kite under Gilead, which are the first CAR-T therapies in human history. CAR-T therapies targeting solid tumors are also under development, such as HER2, EGFR, and Claudin18.2 targets.
TCR-T cell therapy
TCR-T cell therapy is a genetically engineered T cell therapy that involves introducing T cell receptors (TCRs) that recognize specific tumor antigens into T cells, allowing them to specifically identify and attack tumor cells. TCRs are capable of recognizing surface or intracellular/nuclear proteins presented by MHC molecules, thereby enhancing T cells' tumor-killing ability.
Application: TCR-T therapy shows potential for use in treating blood-based cancers as well as solid tumor types. In particular, for some solid tumors that are difficult to treat with CAR-T therapy, such as melanoma and synovial sarcoma, TCR-T therapy may have unique advantages. This therapies commonly target NY-ESO-1, MAGE-A3, and KRAS mutations.
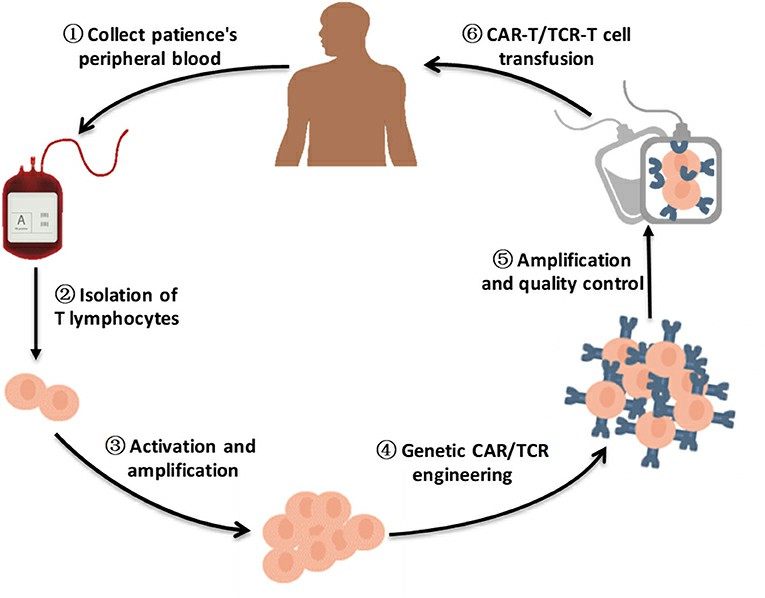 Fig. 1. A brief flow chart of engineered-T cell therapy (Zhao L and Cao YJ, 2019).
Fig. 1. A brief flow chart of engineered-T cell therapy (Zhao L and Cao YJ, 2019).
TIL therapy
Tumor - infiltrating lymphocytes (TILs) are isolated from the patient's tumor tissue. These lymphocytes have already naturally infiltrated and exerted a killing effect in the tumor microenvironment. After in vitro expansion, the TIL cells are re-infused into the patient's body to further exert anti-tumor effects.
Application: TIL therapy is currently mainly applicable to the treatment of solid tumors, such as melanoma, lung cancer, breast cancer and cervical cancer. In particular, melanoma TIL therapy has achieved a high objective response rate (ORR) in clinical trials. Since TIL cells can recognize and kill a variety of tumor antigens, they have unique advantages in the treatment of solid tumors.
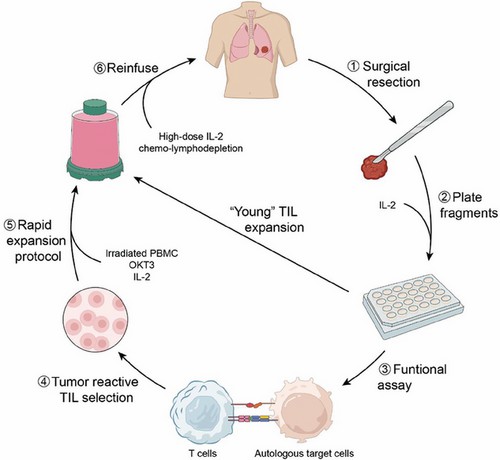 Fig. 2. TIL Production and Administration (Hu W, Bian Y, et al., 2024).
Fig. 2. TIL Production and Administration (Hu W, Bian Y, et al., 2024).
NK cell therapy
Natural killer (NK) cells extracted from patients or donors can be expanded and activated in vitro and then re-infused into the patient's body. They can attack tumor cells with their inherent non-specific killing activity. Natural killer (NK) cells function independently of tumor-specific recognition and remain active without being blocked by the major histocompatibility complex (MHC) present on the cell surface.
Application: It is suitable for the treatment of a variety of solid tumors and hematological malignancies.
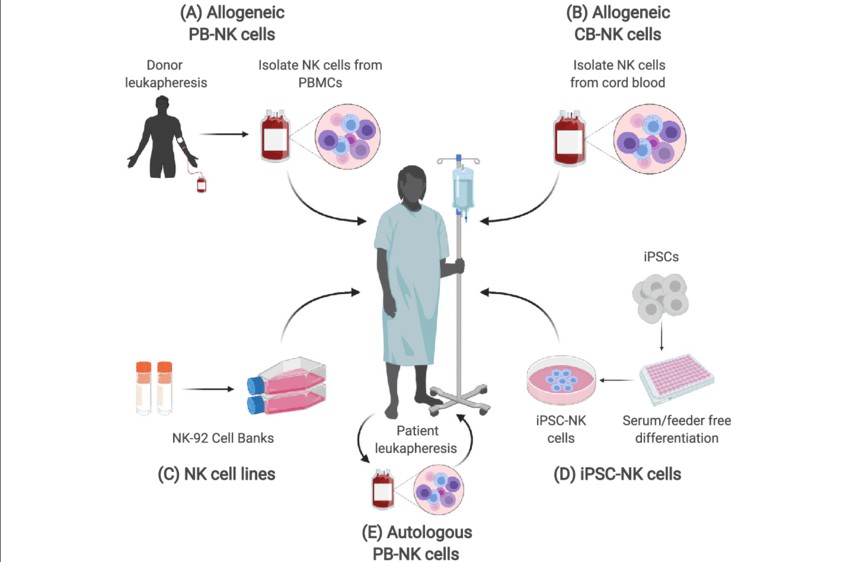 Fig. 3. Sources of NK cells (Shankar K, Capitini CM, et al., 2020).
Fig. 3. Sources of NK cells (Shankar K, Capitini CM, et al., 2020).
Dendritic cell (DC) vaccine therapy
The patient's dendritic cells are co-cultured with tumor antigens in vitro to mature and load tumor antigens, and then re-infused into the patient's body to induce a specific T cell immune response.
Application: The Sipuleucel-T vaccine has been approved by the FDA for the treatment of prostate cancer, which can prolong the median survival of patients by 4.1 months. In acute myeloid leukemia, DC vaccines combined with chemotherapy reduce the recurrence rate by about 30%.
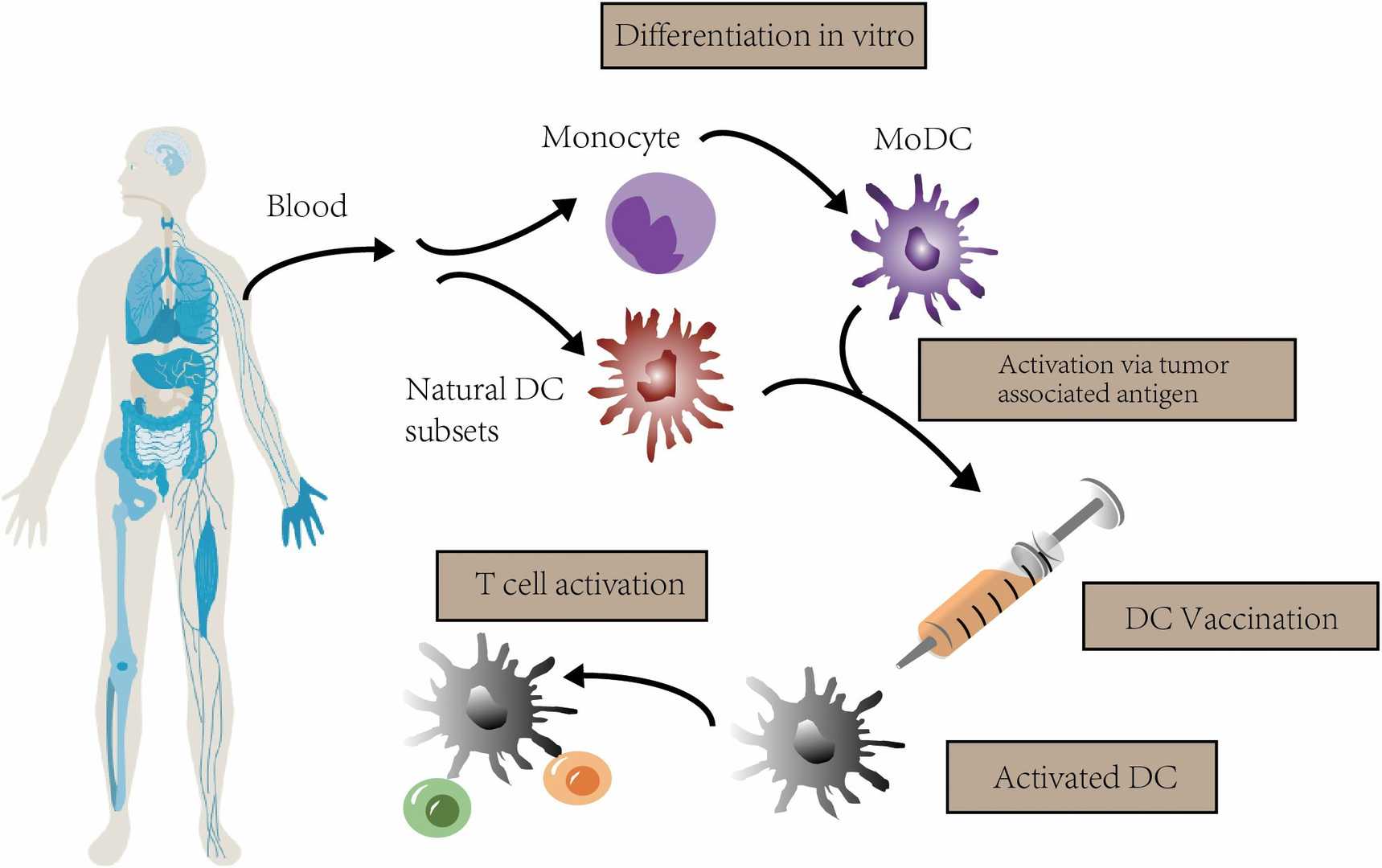 Fig. 4. The concept of DC vaccination. In clinical trials, autologous monocytes are commonly used as the source of DC vaccines with differentiation and maturation in vitro (Zheng Y, Ma X, et al., 2023).
Fig. 4. The concept of DC vaccination. In clinical trials, autologous monocytes are commonly used as the source of DC vaccines with differentiation and maturation in vitro (Zheng Y, Ma X, et al., 2023).
γδ T cell therapy
γδ T Cell Therapy γδ T cells are a subgroup of T cells that have their own unique T cell receptors (TCRs) and can recognize and kill tumor cells and infected cells. By expanding and activating γδ T cells in vitro and then re-infusing them into the patient's body, their anti-tumor and anti-infection functions can be enhanced.
Comparison of immune cell therapies
| Type of Therapy | Advantages | Challenges |
| CAR - T Cell Therapy |
|
|
| TCR - T Cell Therapy |
|
|
| TIL Therapy |
|
|
| NK Cell Therapy |
|
|
| Dendritic Cell (DC) Vaccine Therapy |
|
|
Stem Cell Therapy
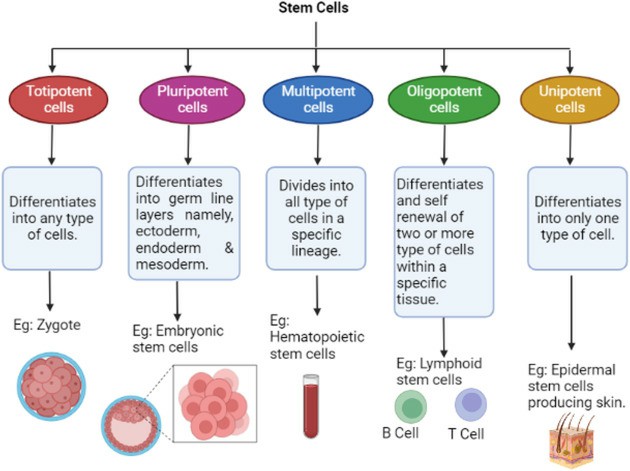 Fig. 5. Represents the classification of stem cells (Rezaei Z, 2024).
Fig. 5. Represents the classification of stem cells (Rezaei Z, 2024).
Hematopoietic stem cell therapy
Hematopoietic stem cells (HSCs), primarily located in bone marrow, have the ability to differentiate into various types of blood cells, including red blood cells, white blood cells, and platelets. The transplantation of HSCs (i.e., bone marrow transplantation) is a common treatment for hematological malignancies (such as leukemia and lymphoma) and some immune deficiency diseases.
Embryonic stem cell therapy
Embryonic stem cells are derived from the inner cell mass of early embryos. These cells have high differentiation potential and, in theory, have the ability to regenerate all tissues. However, the use of embryonic stem cells in clinical practice is limited due to ethical concerns (involving the use of embryos) and the risk of immune rejection.
Induced pluripotent stem cell therapy
Induced pluripotent stem cells (iPSCs) are cells obtained by reprogramming mature cells (such as skin cells or blood cells) into a state similar to embryonic stem cells. iPSCs bypass the ethical issues associated with embryonic stem cells and, being derived from the patient's own cells, have a lower risk of immune rejection. iPSCs have broad application prospects in regenerative medicine, such as the treatment of macular degeneration and heart failure.
Mesenchymal stem cell therapy
Mesenchymal stem cells (MSCs) are widely distributed in various tissues, such as bone marrow, adipose tissue, and umbilical cord blood. MSCs have the ability to differentiate into several types of cells, such as osteoblasts, chondrocytes, and adipocytes, and also have anti-inflammatory and immune-modulating effects. They have shown promise in the treatment of osteoporosis and osteoarthritis. For instance, in osteoporosis, MSCs can promote bone repair and reduce inflammation, and in osteoarthritis, MSCs can regenerate cartilage, reduce inflammation, and promote the repair of joint tissue.
Neural stem cell therapy
Neural stem cells (NSCs) are found in the central nervous system and have the ability to differentiate into neurons, oligodendrocytes, and astrocytes. NSCs therapy has shown potential in the treatment of central nervous system diseases, such as Parkinson's disease, Alzheimer's disease, and spinal cord injury. However, NSCs therapy is still in the research and clinical trial stage, and its effect on nerve regeneration and functional recovery still needs further study.
Skin stem cell (sscs) therapy
Skin stem cells include epidermal stem cells and hair follicle stem cells, which have the ability to maintain the integrity of the skin and promote wound healing. SSCs therapy has potential application value in the field of burns, chronic wounds, and skin regeneration, but it is still in the stage of clinical research.
Umbilical cord blood stem cell therapy
Umbilical cord blood contains a large number of hematopoietic stem cells and other types of stem cells, which can be used for the treatment of various hematological diseases and immune deficiencies. Umbilical cord blood stem cell transplantation has the advantages of low immunogenicity and relaxed donor matching requirements. Moreover, mesenchymal stem cells in umbilical cord blood also have therapeutic potential and can be used in regenerative medicine.
Multicellular therapy
Multicellular therapy refers to the combination of different types of stem cells or cells for treatment, which can produce synergistic effects and enhance treatment efficacy. For example, in tissue engineering, the combination of MSCs and epidermal stem cells can promote skin regeneration and wound healing. Multicellular therapy has broad application prospects in the fields of regenerative medicine and immunotherapy, but it still needs further research and clinical validation.
| Type of Therapy | Advantages | Challenges |
| Hematopoietic Stem Cell Therapy |
|
|
| Mesenchymal Stem Cell Therapy |
|
|
| Embryonic Stem Cell Therapy |
|
|
| Induced Pluripotent Stem Cell Therapy |
|
|
| Neural Stem Cell Therapy |
|
|
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| CAR-T Preclinical Characterization in vivo | CAR-T preclinical characterization is a challenging but necessary step before CAR-T medical products approval. Creative Bioarray provides "one-stop" service for your scientific research, comprehensive and reliable assay results are guaranteed. |
| Immune cell therapy | Creative Bioarray provides high quality GMP clinical products for your needs in immune cell therapy. |
| Stem cell therapy | Creative Bioarray provides high quality GMP clinical products for your needs in stem cell therapy. |
References
- Zhao L, Cao YJ. Engineered T Cell Therapy for Cancer in the Clinic. Front Immunol. 2019. 10:2250.
- Hu W, Bian Y, et al. TIL Therapy in Lung Cancer: Current Progress and Perspectives. Adv Sci (Weinh). 2024. 11(46):e2409356.
- Shankar K, Capitini CM, et al. Genome engineering of induced pluripotent stem cells to manufacture natural killer cell therapies. Stem Cell Res Ther. 2020. 11(1):234.
- Zheng Y, Ma X, et al.Dendritic cell vaccine of gliomas: challenges from bench to bed. Front Immunol. 2023. 14:1259562.