How to Begin with Multiplex Immunohistochemistry (mIHC)
The traditional approach, immunohistochemistry (IHC), has been a popular tool in the pathology and biomedical research fields to visualize specific proteins in tissues. However, the conventional IHC approach has a few major shortcomings. The detection of more than one or two markers in a single round of experiments is not possible, resulting in low throughput. The spatial information is lost and signal precision is limited, making it a suboptimal choice to study the complex biological systems with multiple cellular interactions and pathways.
Enter multiplexed immunohistochemistry (mIHC), a powerful advancement that overcomes these limitations. mIHC allows for the simultaneous detection of multiple protein markers within a single tissue section, preserving spatial context and increasing signal precision. And it has found widespread use in various fields including immuno-oncology, drug development, and biomarker research.
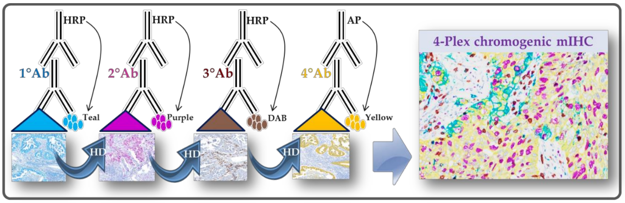
Understanding the Principles of Multiplexed
At its core, mIHC builds upon conventional IHC but introduces advanced labeling and imaging strategies that allow multiple targets to be visualized within the same tissue section without signal overlap or loss of specificity.
Labeling Technologies
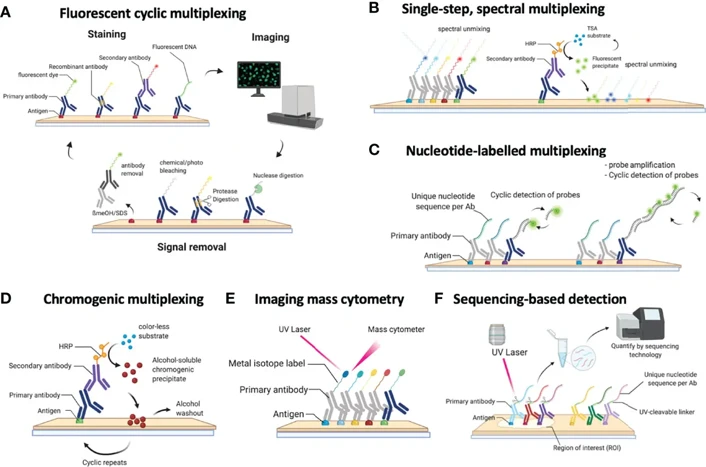
- Fluorescent Multiplexing: This can be achieved by using spectrally unique fluorophores, or with a tyramide signal amplification (TSA) system. When using TSA, multiple antibodies can be labeled sequentially, each with a distinct fluorophore.
- Metal-based Labeling: Techniques like Imaging Mass Cytometry (IMC) use metal-conjugated antibodies that are detected via mass spectrometry. This allows for high-dimensional, interference-free imaging.
- Cyclic and Iterative Methods: Cyclic immunofluorescence and iterative staining and stripping methods allow repeated rounds of staining and imaging, achieving panels with 10-40 markers or more.
Signal Amplification and Detection
The tyramide signal amplification (TSA) system is commonly used for fluorescence-based mIHC. In TSA, an enzyme-mediated reaction is used to covalently deposit fluorophore-labeled tyramides at the site of target antigens, resulting in robust, localized signals even for low-abundance proteins. Antibodies are stripped away after each staining round, but the deposited signal remains in place for the next round.
Preservation of Spatial Context
Unlike flow cytometry or Western blotting, mIHC maintains the spatial relationships between cells and structures within the tissue. This feature allows researchers to not only measure marker expression but also understand how different cell populations interact within their native microenvironment - a critical factor in cancer immunology and tissue pathology.
Planning Your First mIHC Experiment
Step 1: Define Your Biological Question
Every experiment should be driven by a specific scientific question. Consider the biological process or cell type you are interested in, such as immune checkpoint expression in the TME or stromal-immune cell profiling. This will determine the choice of markers and the imaging strategy.
Tip: Focus on a defined hypothesis. A well-formulated question ensures efficient panel design and interpretable results.
Step 2: Select and Validate Antibodies
One of the most important steps in mIHC is selecting a panel of compatible antibodies.
- Use highly specific, validated antibodies from reputable sources.
- Ensure that antibodies come from different host species or isotypes to reduce cross-reactivity.
- For sequential staining, validate each antibody individually under single-plex conditions before multiplexing.
- Fluorescent crosstalk and non-specific binding can be reduced by optimizing antibody concentrations, washing steps, and blocking conditions.
Common challenge: Signal overlap between fluorophores.
Solution: Use fluorophores with minimal spectral overlap, and apply spectral unmixing during analysis.
Step 3: Optimize Tissue Preparation
Tissue handling can significantly affect staining quality. Formalin-fixed paraffin-embedded (FFPE) tissues are most commonly used due to their stability and morphological preservation.
- Fixation time should be consistent; over-fixation can mask epitopes, while under-fixation can compromise morphology.
- Antigen retrieval (heat- or enzyme-based) is essential for exposing masked epitopes, but conditions must be carefully tuned to prevent tissue damage.
- Section thickness (usually 4-5 µm) and storage duration can also affect signal intensity.
For frozen tissues, ensure proper cryoprotection and fixation to preserve antigenicity and tissue integrity.
Step 4: Establish the Staining Workflow
Typical mIHC workflows involve several key steps:
- Sequential antibody staining - applying and detecting one antibody at a time.
- Signal amplification - enhancing the detected signal, often via TSA.
- Antibody stripping - removing bound antibodies without disrupting deposited signals.
- Repetition - cycling through additional markers until the full panel is completed.
Automated staining systems can streamline this process, reduce variability, and improve reproducibility, especially for larger studies.
Step 5: Image Acquisition and Analysis
High-quality imaging is crucial to capture and quantify multiplexed signals accurately.
- Multispectral imaging systems can separate overlapping fluorescence spectra, allowing precise signal deconvolution.
- High-resolution scanners are also available for whole-slide imaging of large tissue sections.
- AI-assisted image analysis software can segment individual cells, quantify marker expression, and assess spatial relationships.
Pro tip: Always include proper controls - single-stained, no-primary, and isotype controls - to validate staining specificity and analytical thresholds.
Step 6: Troubleshooting and Optimization
Even well-designed mIHC experiments require iterative refinement.
Common issues include:
- Uneven staining or background noise: Optimize blocking buffers and washing steps.
- Loss of signal after multiple cycles: Adjust stripping conditions and verify fluorophore stability.
- Spectral overlap: Re-design panels with non-overlapping emission spectra or apply computational unmixing algorithms.
Systematic optimization ensures reproducibility and robust quantitative data.
Key Applications of mIHC
Multiplex IHC has become absolutely necessary for profiling the tissue microenvironment in situ. Here are some major applications of mIHC in which we are actively involved:
- Immuno-oncology: Profiling the tumor immune contexture including quantification of density and spatial organization of different immune cell populations (e.g., cytotoxic T-cells, Tregs, etc) and checkpoint molecules for immune therapy prediction.
- Drug Development: Mechanism of action studies in preclinical research and identification of predictive/pd biomarkers in clinical trials.
- Biomarker Discovery & Disease Research: Investigating complex cell interactions in neurodegenerative, autoimmune, infectious and many other diseases where single marker approaches have been inadequate and a comprehensive understanding of the cell-cell and molecular pathways is needed.
In summary, the multiplex IHC journey has a substantial upfront investment. But once on the journey, the return is second to none. It allows one to view tissue biology in multiple dimensions with high resolution that cannot be achieved by other methods.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
|---|---|
| Immunohistochemistry (IHC), Immunofluorescence (IF) Service | Creative Bioarray offers a comprehensive IHC service from project design, marker selection to image completion and data analysis. |
| Histology Services | Creative Bioarray offers tissue processing, embedding, sectioning, and staining, along with set of histological examination services. |
References
- Hofman P, Badoual C, et al. Multiplexed Immunohistochemistry for Molecular and Immune Profiling in Lung Cancer-Just About Ready for Prime-Time? Cancers. 2019; 11(3):283.
- Bosisio FM, Van Herck Y, et al. Next-Generation Pathology Using Multiplexed Immunohistochemistry: Mapping Tissue Architecture at Single-Cell Level. Front Oncol. 2022; 12:918900.