From Collection to Cure: How ACT Works in Cancer Immunotherapy
Traditional cancer treatment, such as chemotherapy and radiotherapy, can be considered as a "double-edged sword". On the one hand, they eliminate cancer cells and suppress tumor growth; on the other hand, they damage the normal tissues and cause serious side effects, such as decreased immunity and poor quality of life for the patients. In recent years, adoptive cell therapy (ACT) with "precise targeting and long-lasting immunity" has brought new hope to cancer treatment.
What is ACT?
ACT is a personalized cancer treatment that aims to boost anti-tumor immune responses by collecting patient's own or donor's immune cells, activating, genetically modifying and expanding in vitro, and then infusing the processed immune cells back into the patient. ACT operates on the strategy of "using strength against strength" by activating the body's natural immune cells to identify and destroy cancer cells instead of using radiation or medication treatments.
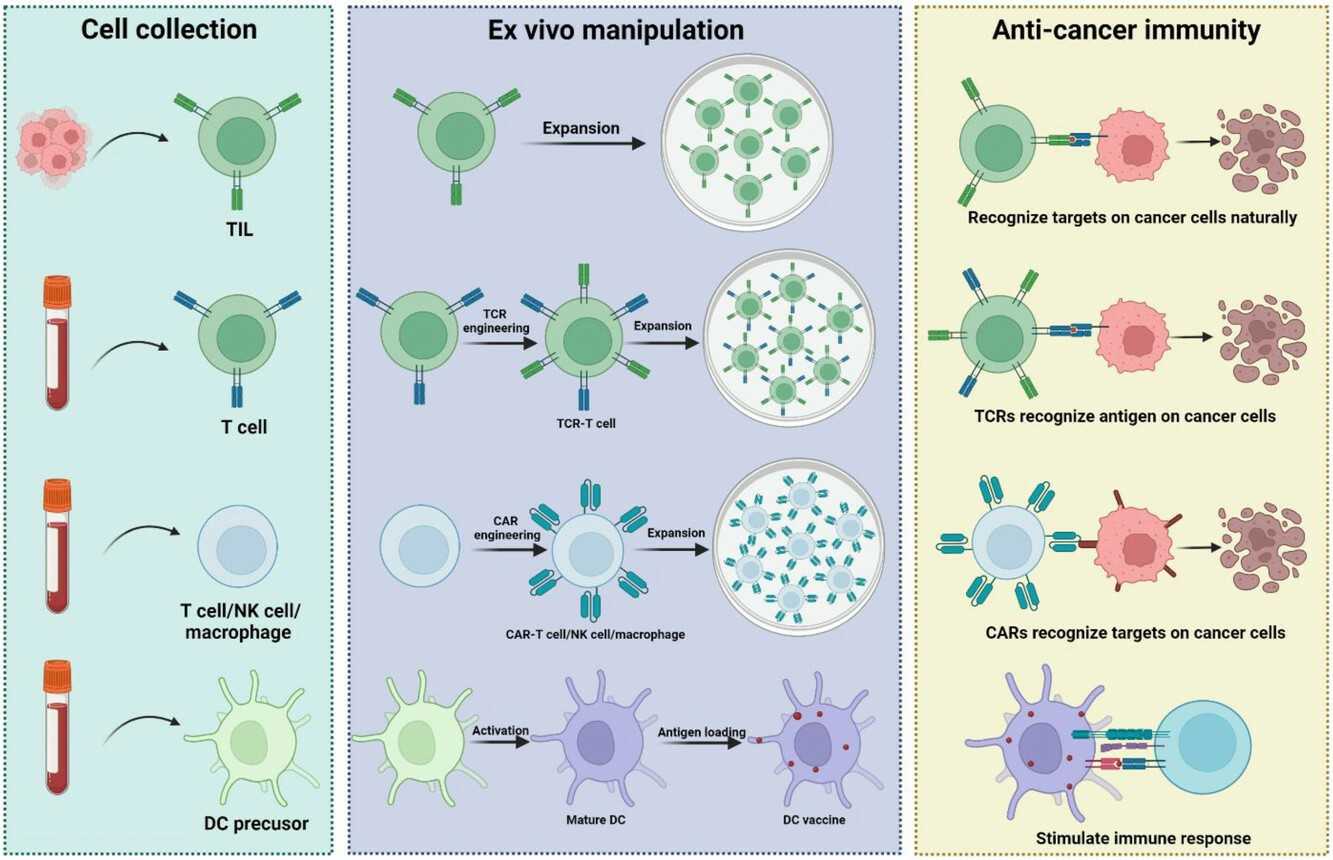 Fig. 1. Diverse ACT platforms (Du S, Yan J, et al., 2019).
Fig. 1. Diverse ACT platforms (Du S, Yan J, et al., 2019).
How to perform ACT?
The treatment process of ACT can be summarized into four major steps, each of which needs to be carefully controlled for the therapeutic effect:
1. Cell collection
ACT requires a high-quality immune cell source. Typically, these cells are sourced from the patient's blood, lymph nodes, or tumor tissues. In some situations, allogeneic immune cells can also be collected; however, autologous immune cells are more appropriate for personalized treatment. Common cell collection methods include:
- Peripheral Blood Apheresis
Method: Peripheral blood is drawn from the patient and processed through a blood cell separator (e.g., Fenwal CS-3000) to separate and collect specific components (such as T cells, NK cells, or mononuclear cells) of blood cells.
Advantages: This method is non-invasive, can be repeated multiple times, and is more suitable for patients with long-term treatment needs. It also quickly isolates and enriches target cells, improving treatment efficiency.
Challenges: In some patients (e.g., late-stage cancer patients), the collected T cells may be exhausted, which may affect subsequent T cell expansion.
- Tumor-Infiltrating Lymphocytes (TILs) Extraction
Method: Tumor-infiltrating lymphocytes (TILs) are a type of T cells isolated from the tissue of solid tumors with anti-tumor activity. TILs are usually extracted from tumor tissues after surgical resection and then expanded in vitro for reinfusion. This method is more suitable for patients with solid tumors such as melanoma and lung cancer.
Advantages: TILs have a natural ability to recognize and kill tumor cells with high specificity. Compared to traditional chemotherapy, TIL therapy has fewer side effects.
Challenges: The method requires surgical resection of tumor tissue, which is invasive and difficult to obtain. Additionally, during the in vitro expansion process of TILs, TILs may also experience exhaustion or functional decline.
- Hematopoietic Stem Cell Source
Method: Immune cells are extracted from healthy donors or umbilical cord blood and then undergo gene editing (e.g., CAR-T or TCR-T modification) for allogeneic therapy. This method is more suitable for the development of universal CAR-T (UCAR-T).
Advantages: (1) Versatility: Donor-derived immune cells can be widely used for allogeneic therapy, reducing the dependence on individual matching. (2) Safety: Donor-derived cells can undergo strict screening and processing to ensure safety.
Challenges: Allogeneic transplantation may cause immune rejection responses and may require immunosuppressive therapy. In addition, the gene editing technology itself is still in the research and development stage and faces certain technical challenges.
2. Cell modification
In vitro modification is the core step of ACT, enhancing the anti-tumor activity of immune cells through gene editing or cytokine stimulation:
- Gene Modification Technology
CAR-T Therapy: Introduces a gene encoding the CAR into T cells, allowing them to express receptors that recognize surface antigens on cancer cells. For example, CD19 antigen-directed CAR-T therapy for B-cell lymphoma has a complete remission rate of 60%-90% in clinical trials.
TCR-T Therapy: It engineers T cells to express certain T-cell receptors (TCRs), which can specifically recognize intracellular tumor antigens (such as NY-ESO-1), and is mainly used for the treatment of solid tumors.
- Cytokine Activation
LAK Cell Therapy: It uses interleukin-2 (IL-2) to stimulate peripheral blood lymphocytes to obtain non-specific killing activity, but the use of a high dose of IL-2 can cause serious side effects.
CIK Cell Therapy: It co-cultures a variety of cytokines such as interferon-γ, IL-2 and CD3 monoclonal antibody to obtain a heterogeneous cell population with T cell and NK cell characteristics, which is mainly used for early-stage tumor adjuvant therapy.
3. Cell expansion
After modification, immune cells need to be expanded in specific culture conditions to increase their numbers to a sufficient therapeutic dose.
- Culture System
Cell expansion is typically carried out in an in vitro culture system that provides a suitable growth environment and nutritional conditions to promote cell proliferation and functional maintenance.
Culture Medium: The culture medium is fundamental for cell expansion, usually containing a basic medium (such as RPMI-1640 or DMEM) and various cytokines to support cell growth and function. Commonly used cytokines include:
IL-2: A core factor for T-cell expansion that promotes cell proliferation and survival by activating the JAK-STAT pathway. For example, in CAR-T expansion, the IL-2 concentration is usually 300-1000 IU/mL, but high doses may cause T-cell exhaustion.
IL-7/IL-15: "Second-generation" factor combinations that can induce central memory T cell (Tcm) differentiation, enhancing cell persistence. For instance, IL-15 can reduce PD-1 expression on CAR-T cells, delaying functional exhaustion.
Other factors: Such as IL-12 (enhancing cytotoxicity), IL-21 (promoting B cell killing), etc., dynamically adjusted based on treatment goals.
- Bioreactor
For large-scale cell expansion, bioreactors are typically used for cultivation. Based on operational mode and control precision, bioreactors can be categorized into:
Wave Bioreactor: Reduces shear stress through periodic wave motion, suitable for shear-sensitive cells (like CAR-T cells).
Closed-Loop System: Such as GMP-grade bioreactors with automatic control, sterile operation, and real-time monitoring, suitable for large-scale production in compliance with GMP standards.
- Quality Control
Expanded immune cells need to undergo multi-dimensional quality testing to ensure they meet clinical treatment standards, with key indicators including:
Cell Viability: Determined by flow cytometry or cell counting methods, generally requiring a cell viability rate of >90%.
CAR/TCR Expression Efficiency: Determined by flow cytometry to ensure that the transgenic cells have sufficient anti-tumor activity. Generally, CAR/TCR expression efficiency should be >70%.
Sterility Testing: Ensuring that there is no microbial contamination, generally through the sterility testing of culture media and cells (such as PCR, culture tests), to prevent cell infection.
Cell Purity: Determined by flow cytometry or immunofluorescence to ensure the purity of target cells (such as CAR-T cells).
Cell Function Testing: Assessed through in vitro cytotoxicity assays or cytokine release tests (such as IFN-γ, TNF-α) to determine the anti-tumor activity.
4. Cell reintegration
Cell reinfusion refers to the process of reintroducing the expanded immune cells back into the patient's body. Prior to reinfusion, the patient often undergoes some pre-treatment (such as chemotherapy or radiotherapy) to reduce the number of immunosuppressive cells in the body and create a more conducive environment for the survival of the reinfused immune cells. Typically, the reinfusion procedure involves intravenous administration which resembles a standard blood transfusion. After the reinfusion of the cells, the immune cells can rapidly expand in the patient's body and actively seek out and attack cancer cells. Doctors will continuously assess patient responses and adverse effects throughout treatment to make necessary modifications to the treatment plan.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| CAR-T Preclinical Characterization in vivo | CAR-T preclinical characterization is a challenging but necessary step before CAR-T medical products approval. Creative Bioarray provides "one-stop" service for your scientific research, comprehensive and reliable assay results are guaranteed. |
| Immune cell therapy | Creative Bioarray provides high quality GMP clinical products for your needs in immune cell therapy. |
| Stem cell therapy | Creative Bioarray provides high quality GMP clinical products for your needs in stem cell therapy. |
Reference
- Du S, Yan J, et al. Adoptive cell therapy for cancer treatment. Exploration (Beijing). 2023. 3(4):20210058.