Cell-Based High-Throughput Screening Techniques
Cell-Based High-Throughput Screening (CB-HTS) is an advanced and highly effective experimental technology that has gained widespread use in the field of drug discovery and biomedical research. By utilizing automation technology, CB-HTS allows for the rapid and efficient screening of a large number of compounds, identifying their potential effects on specific cellular processes or disease models. This technology not only accelerates the drug discovery process but also provides valuable insights into the underlying mechanisms of biological processes, making it a crucial tool in modern biomedical research.
Applications
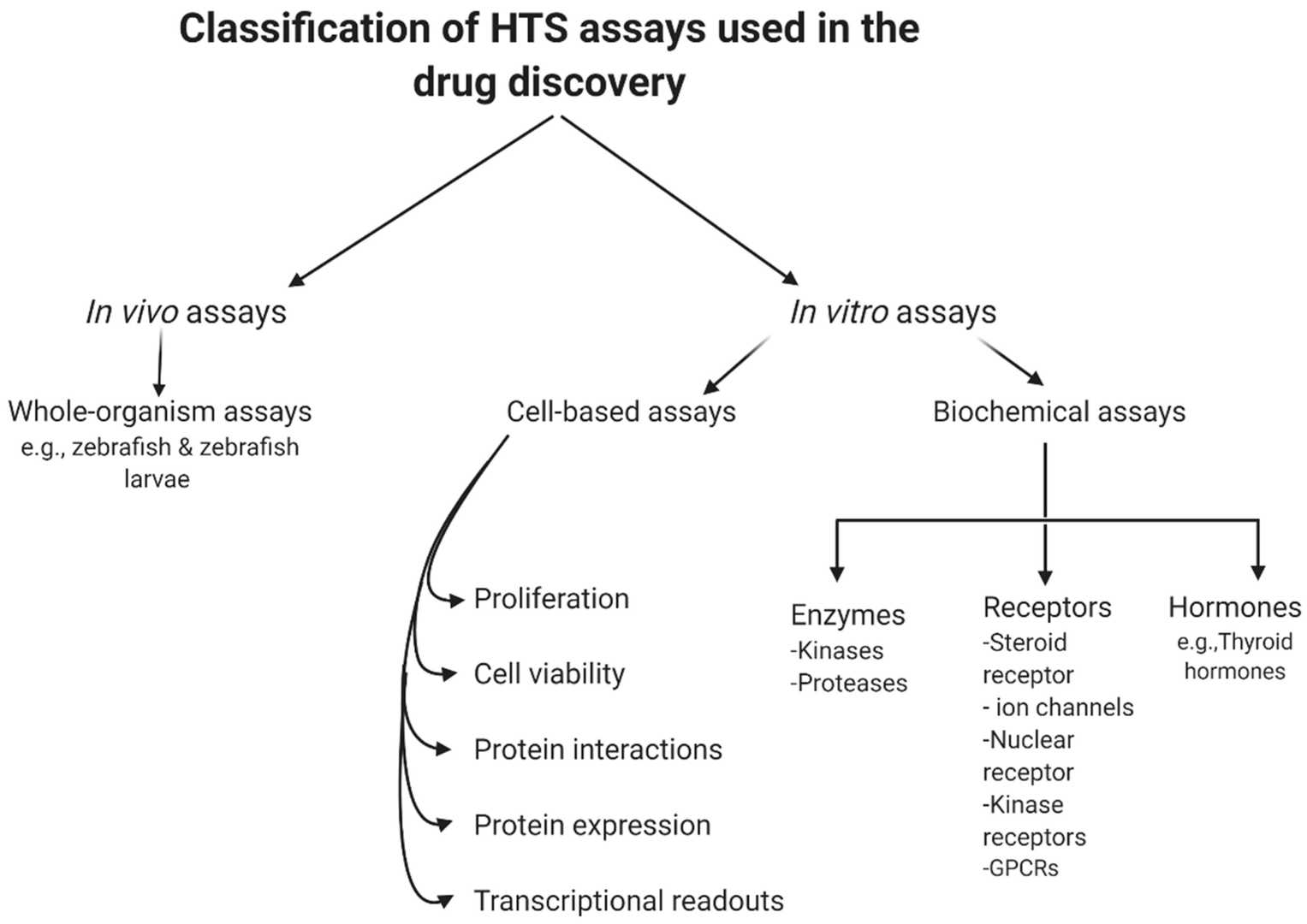 Fig. 1. General classification of high-throughput screening (HTS) assays (Aldewachi H, Al-Zidan RN, et al. 2021).
Fig. 1. General classification of high-throughput screening (HTS) assays (Aldewachi H, Al-Zidan RN, et al. 2021).
- Drug Discovery: CB-HTS is commonly used in the early stages of drug development to rapidly identify bioactive candidate molecules from large compound libraries. By screening these compounds at the cellular level, it enables the identification of potential drug targets and the optimization of lead compounds.
- Basic Biological Research: This technology is extensively used in fundamental biological studies to explore complex cellular processes such as gene expression regulation, signal transduction pathways, and protein-protein interactions. It allows for a more comprehensive understanding of cellular mechanisms and facilitates the discovery of new biological phenomena and the development of novel therapeutic approaches.
- Biotechnology Applications: CB-HTS has wide-ranging applications in the biotechnology industry, including the development of biotherapeutics, the optimization of cell-based assays for diagnostic purposes, and the study of cellular responses to various environmental factors.
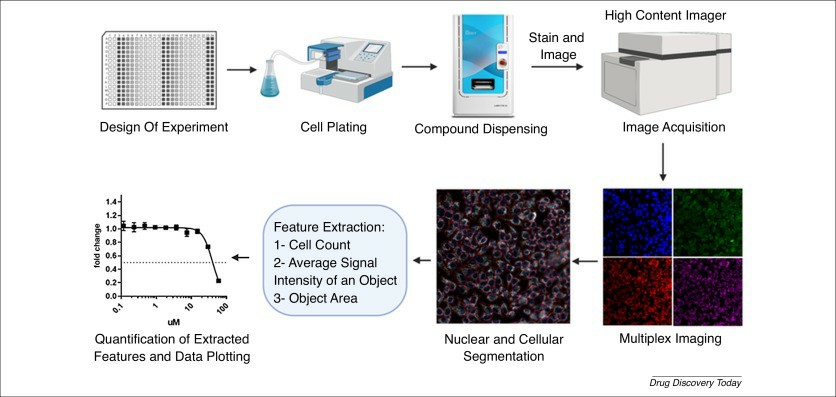 Fig. 2. High-Throughput Screening: today's biochemical and cell-based approaches (Blay V, Tolani B, et al. 2020).
Fig. 2. High-Throughput Screening: today's biochemical and cell-based approaches (Blay V, Tolani B, et al. 2020).
Detection Methods
Reporter gene assay
Reporter gene assays involve introducing an exogenous gene (e.g., luciferase, β-lactamase, GFP) into cells and monitoring its expression level to reflect the activity of a target gene's regulation or a signaling pathway. It is commonly used in studies of gene expression regulation, drug screening, signal transduction, etc. Its advantages are high sensitivity and simple operation, but it needs to construct stable cell lines and may affect the expression of endogenous genes.
Fluorescence/bioluminescence resonance energy transfer (F/BRET)
Fluorescence Resonance Energy Transfer (FRET) and Bioluminescence Resonance Energy Transfer (BRET) are detection techniques based on changes in intermolecular distance, used to study protein-protein interactions, conformational changes, and signal transduction. In drug screening, this technique is often used to identify the binding of small molecule ligands to target proteins and protein-protein interactions. BRET has no background interference and can be used for real-time detection in living cells, but it is limited to a fixed donor-acceptor pair and requires the construction of fusion proteins.
Calcium mobilization assay
Calcium ions (Ca²⁺) are an important second messenger in cells, and play a role in a variety of physiological processes, including cellular signal transduction, muscle contraction, secretion of neurotransmitters, etc. Calcium mobilization assays usually detect the change of intracellular Ca²⁺ concentration by fluorescent dyes (such as Fluo-4) or coelenterazine based system. After coelenterazine binds to the apoaquorin protein (APO), the binding of Ca²⁺ induces an oxidation reaction, which causes blue light to be released, so that Ca²⁺ can be quantitatively detected. In drug screening, this technique is often used to evaluate a compound's effects on calcium signaling pathways.
Label-free detection
Label-free detection technologies do not require fluorescent or radioactive labeling of biological molecules. Instead, they detect changes in the physical or electrical properties of cells, such as membrane potential, dielectric properties, or responses of optical biosensors. Examples of label-free detection methods include Cellular Dielectric Spectroscopy (CDS) and Surface Plasmon Resonance (SPR). Label-free detection technologies can be used to detect a wide range of cellular processes, such as membrane potential changes, cell adhesion, and cell migration. These methods have the advantage of high sensitivity and low background, making them useful in drug screening.
Membrane potential detection
Membrane potential refers to the electrical potential difference between the inside and outside of the cell membrane. It is an important parameter that reflects cellular excitability and ion channel activity. Membrane potential can be detected using various methods, including fluorescent dyes (e.g. DiBAC4) or electrode-based techniques. In drug screening, membrane potential detection can be used to determine the inhibitory effect of drugs on ion channels. This method has been used in studies of neural signaling, muscle contraction, and ion channel regulation. For example, it can be used in screening anti-arrhythmic drugs. The concentration of the probe needs to be optimized in order to avoid background.
Patch clamp technique
Patch clamp is an electrophysiological technique used to study the activity of ion channels in the membrane of single cells. By creating a small seal (or patch) on the cell membrane and recording changes in the ionic current flowing through the patch, this method allows precise measurement of the opening and closing state of the ion channels. The patch clamp technique is widely used in neuroscience, pharmacology, and cell biology to study ion channel function and mechanisms of action of drugs. Patch clamp offers single-channel resolution and is considered the "gold standard" for ion channel studies.
Flow cytometry
Flow cytometry is a high-throughput technique for analyzing multiple parameters of individual cells, including size, granularity, surface antigens, DNA content, cell cycle status, and apoptosis rate. Cells are hydrodynamically focused into a single file and passed through a laser beam. The scattered light and fluorescence signals emitted by the fluorescent dyes excited by the laser are detected and used to classify cells and sort them. Flow cytometry is widely used in immunology, oncology, and drug screening. Key advantages of this technique are multiplexed analysis and high speed (up to tens of thousands of cells per second). The method requires the preparation of single-cell suspensions and does not allow long-term observation.
High-content screening (HCS)
High-content screening (HCS) is a high-throughput screening technology that combines automated microscopy and image analysis for studying complex biological processes, such as cell morphology, organelle distribution, apoptosis, and cell migration. HCS is usually performed on confocal microscopes or live-cell imaging systems (such as ImageStreamX) that allow generating images of several cells simultaneously and performing quantitative analysis. In drug screening, HCS can be used to assess the effect of compounds on cell morphology and function. This technique also allows for multiplexed target and parameter analysis. The main challenges associated with HCS are the complexity of the image analysis algorithms and the large amount of generated data.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| Cell-based Screening and Profiling Services | Speed up your drug discovery with Creative Bioarray's cell-based screening and profiling services. |
References
- Aldewachi H, Al-Zidan RN, et al.High-Throughput Screening Platforms in the Discovery of Novel Drugs for Neurodegenerative Diseases. Bioengineering. 2021. 8(2):30.
- Blay V, Tolani B, et al. High-Throughput Screening: today's biochemical and cell-based approaches. Drug Discov Today. 2020. 25(10):1807-1821.