Caspase Activity Assay
Background
As the central components of apoptosis response, caspases are a class of aspartate-specific cysteine proteases, which can be divided into three functional categories, initiator caspases (caspase-2, -8, -9, -10), effector caspases (caspase-3, -6, -7), and caspases involved in inflammatory cytokine process (caspase-1, -4, -5, 11, -12). All caspases are synthesized in cells as inactive zymogens containing variable length pro-domains, followed by a large (20 kDa) and a small (10 kDa) subunit.
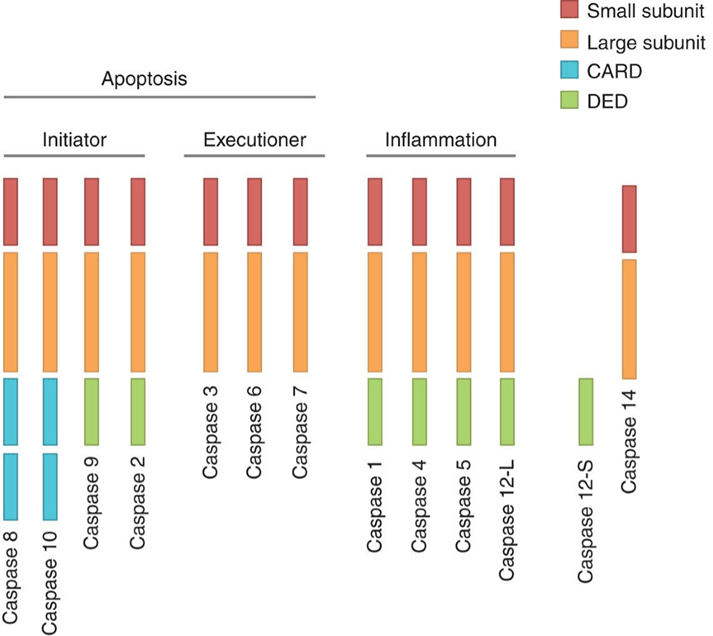 Figure 1. Domain structure of human caspases.
Figure 1. Domain structure of human caspases.
The apoptotic programs are distinguished by either the intrinsic or the extrinsic pathway, depending on the adapters and initiator caspases involved.
The intrinsic pathway is activated by a wide range of death stimulus generated within the cells, such as oncogene activation and DNA damage. Intrinsic apoptosis is also mediated by mitochondria because several proteins (cytochrome C) are released from the mitochondrial intermembrane space into the cytoplasm. Cytochrome C activates the protein APAF1 in the cytoplasm, which mediates the activation of caspase-9 and triggers the cascade activation of caspase.
The extrinsic pathway is triggered by the binding of extracellular ligands (FasL and TNF-alpha) to cell surface death receptors (FasR and TNFR). The binding of death receptors causes the recruitment of monomeric procaspase-8, which results in dimerization and activation.
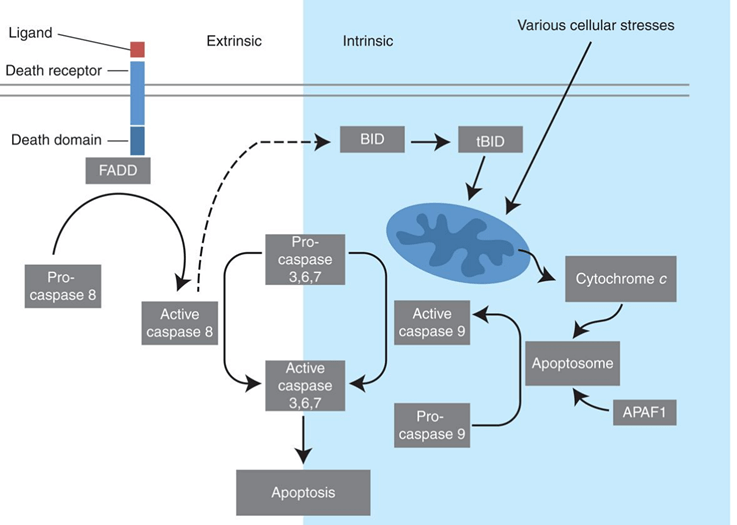 Figure 2. Extrinsic and intrinsic pathways of apoptosis.
Figure 2. Extrinsic and intrinsic pathways of apoptosis.
Several caspases act as key mediators in innate immune responses rather than pro-apoptotic factors such as caspase-1, -4, -5, and -12. These inflammatory caspases, like their pro-apoptotic counterparts, are produced as inactive proteases in resting cells. Only external or internal stimulations can promote the assembly of leucine-rich repeat-containing (NLR) proteins (similar to the assembly of APAF1 in apoptosome). The activation of NLR permits the recruitment of inflammatory caspases, typically caspase-1, and then inflammatory caspases activated through the formation of a cytosolic complex termed the inflammasome.
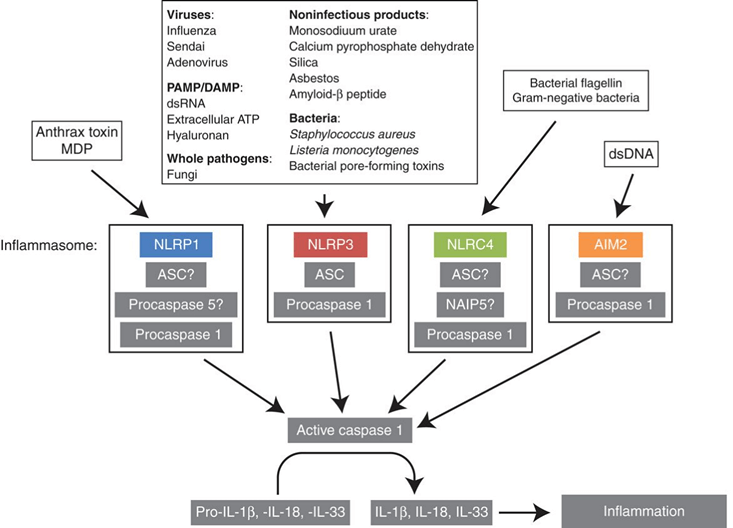 Figure 3. Signalling and composition of inflammasomes.
Figure 3. Signalling and composition of inflammasomes.
A prominent feature of the early stages of apoptosis is the activation of caspase, which is involved in the cleavage of protein substrates and in the subsequent disintegration of the cell. Creative Bioarray offers a series of caspase assay kits that allow to detect active caspases in apoptosis cells. Caspase-3 is the most widely used detection method.
Caspase-3 Activity Assay (Colorimetric)
- About this assay
Caspase-3 initiates apoptosis or other cellular processes in mammalian cells. As a simple and convenient analysis method, the caspase-3 activity assay determine the activity of caspase by recognizing the sequence DEVD. This assay is based on spectrophotometric detection of chromophore p-nitroanilide (pNA) cleaved from the labeled substrate DEVD-pNA. Light emission of the pNA can be quantified using a spectrophotometer or a microtiter plate reader at 400-405 nm. Comparing the pNA absorbance of the treated samples and untreated controls allow determination of the fold increase in caspase-3 activity.
- Cell set up
a) For suspension cells: centrifuge and then suspend the cell pellet in culture medium at 80,000 cells/well with a 96-well plate.
b) For adherent cells: seed cells in a 96-well plate at the density of 20,000 cells/well in growth medium.
c) Seeding density will depend on cell type and should be evaluated to determine the optimal cell density for apoptosis induction.
d) Induce cell apoptosis according your experimental protocol, and concurrently incubate a control without induction.
e) We recommend that all treatments in triplicate.
- Reagent preparation
a) Thaw out all components at room temperature just before experiment.
b) Prepare enough caspase-3 assay loading solution by adding the DEVD-pNA substrate and DTT reagent according to your instructions.
c) If you are not using all of the caspase-3 substrate at one time, we recommend storing the unused components at -20°C.
- Assay procedures
a) Centrifuge and re-suspend cells in chilled cell lysis buffer.
b) Incubate on ice for 10 minutes.
c) Add 100 μL/well of caspase-3 assay loading solution (from reagent preparation b).
d) Incubate the loading solution plate at 37°C for 1-2 hours.
e) Measure output (OD 400-405 nm) on a microplate reader, or spectrophotometer using a quartz cuvette.
Caspase-3 Activity Assay (Fluorometric)
- About the assay
The caspase-3 fluorometric assay is based on the fluorogenic substrate N-AcetylAsp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC). In apoptotic cells, activated caspase-3 cleaves the substrate between DEVD and AMC, producing a highly fluorescent AMC that can be detected by a fluorescence reader with excitation at 380 nm and emission between 420-460 nm. The amount of AMC generated is proportional to the number of apoptotic cells in the sample.
- Assay protocol
a) Induce cell apoptosis according your experimental protocol, and concurrently incubate a control without induction.
b) Count cells (depending on the degree of apoptosis)
c) Re-suspend cells in chilled cell lysis buffer (50 μL) and incubate on ice for 10 minutes.
d) Centrifuge at 800 g for 10 minutes.
e) Add reaction buffer (containing 10 mM DTT) and DEVD-AMC substrate (final concentration 50 μM) to each sample and incubate at 37°C for 2 hours.
f) Read samples in a fluorometer equipped with a 380 nm excitation filter and 420-460 nm emission filter.
References
- Riedl SJ, et al.; Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Bio, 2004, 5(11): 897-907.
- David R. M., et al.; Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol, 2013, 5.
Cell Services:
Cell Line Testing and Assays:
Explore Other Options