How to Decide Between 2D and 3D Cell Cultures?
Cell culture techniques play a crucial role in life science research, providing a controlled environment for studying cellular behavior and interactions. Traditionally, two-dimensional (2D) cell cultures have been the primary choice due to their simplicity and ease of use. However, three-dimensional (3D) cell cultures have emerged as a more physiologically relevant model, offering a closer approximation of the complex cellular microenvironment found in vivo.
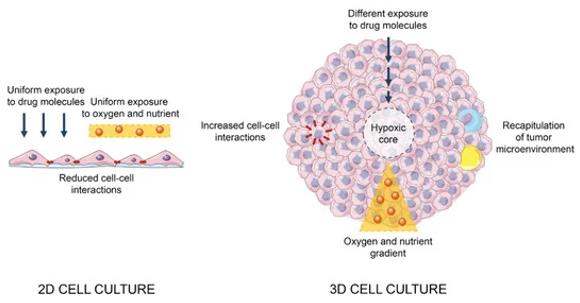 Fig. 1 Main differences between 2D and 3D cell cultures. (Fontana F, et al., 2020)
Fig. 1 Main differences between 2D and 3D cell cultures. (Fontana F, et al., 2020)
Advantages and Limitations of 2D Cell Cultures
2D cell cultures have been the gold standard for many years, primarily due to their simplicity and cost-effectiveness. They involve growing cells on a flat surface, such as tissue culture plates or Petri dishes. However, 2D cell cultures have limitations that restrict their ability to fully recapitulate the complexity of in vivo cellular behavior.
| Advantages | Limitations |
|
|
Reasons for Persevering with 2D Cell Cultures
Despite the limitations, 2D cell cultures continue to be utilized for various applications in life science research. Several reasons contribute to their continued use:
- Established protocols and expertise. Over the years, extensive knowledge and expertise have been accumulated for working with 2D cell cultures, making them a familiar and reliable choice for many researchers.
- Specific research objectives. Certain research questions may require a simplified cellular environment to study specific cellular processes or molecular mechanisms, where 2D cultures can provide valuable insights.
- Comparative studies. Comparing data obtained from 2D cultures with existing literature or historical data facilitates comparisons and allows for consistency in research findings.
Types of 3D Cultures
Three-dimensional cell cultures aim to bridge the gap between traditional 2D cultures and complex in vivo conditions. Various 3D culture systems have been developed to replicate the cellular microenvironment more accurately. Some common types of 3D cultures include:
- Spheroids. Spheroids are three-dimensional aggregates of cells that can be formed by suspension cultures or using specialized platforms. They allow for cell-cell interactions and the formation of gradients within the spheroid, more closely resembling native tissue structures.
- Organoids. Organoids are self-organizing, three-dimensional structures that mimic specific organs or tissues. They are generated from stem cells or tissue-specific progenitor cells and can recapitulate complex tissue architecture and functionality.
- Hydrogels. Hydrogels provide a 3D matrix for cell encapsulation, allowing cells to grow and interact within a biomimetic environment. Hydrogels can be derived from natural or synthetic materials and offer tunable properties to mimic the extracellular matrix (ECM).
Advantages of 3D Cultures
3D cell cultures offer several advantages over their 2D counterparts, making them increasingly popular for many research applications.
- Improved physiological relevance. 3D cultures better mimic the three-dimensional architecture and mechanical cues experienced by cells in vivo, providing a more physiologically relevant environment for studying cellular behavior and responses.
- Enhanced cell-cell interactions. Cells in 3D cultures can interact more closely, allowing for cell signaling, communication, and the formation of complex cellular networks. This enables the study of cell-cell interactions and cellular processes that are crucial for tissue development, homeostasis, and disease progression.
- Tissue-specific functionality. Organoids, a type of 3D culture, can exhibit tissue-specific functionality and recapitulate organ-specific structures and physiological processes. This makes them valuable tools for studying organ development, disease modeling, and personalized medicine approaches.
Deciding Between 2D and 3D Cultures
The decision to use 2D or 3D cell cultures should be based on the specific research objectives and the desired level of physiological relevance. Consider the following factors when deciding between 2D and 3D cultures:
- Research questions. Determine whether the research questions require a simplified cellular environment or a more complex, physiologically relevant model.
- Cell type and tissue. Consider the compatibility of the cell type or tissue with 2D and 3D culture systems. Some cell types may perform better in one system over the other.
- Experimental throughput. Assess the experimental throughput requirements. 2D cultures are generally more amenable to high-throughput screening, while 3D cultures may be more suitable for detailed mechanistic studies.
- Resources and expertise. Evaluate the availability of resources, equipment, and expertise required for both 2D and 3D culture systems. Consider the cost, time investment, and technical capabilities of the research team.
Creative Bioarray Relevant Recommendations
| Service Types | Description |
| 3D-based Services | Creative Bioarray conducts custom 3D-based cell services with comprehensive and advanced 3D culture systems in the field including scaffold-based and scaffold-free cell culture technologies to ensure the best result for your research goals |
| Custom 3D Cell Culture Services | Creative Bioarray has extensive experience in developing highly functional 3D cell culture models that exhibit more physiological relevance. |
| 3D Cell Culture Related Products | Creative Bioarray offers a wide range of 3D cell culture-related products to support your in-house development of 3D models, including products for spheroids formation, 3D cell culture scaffolds, bioreactors, and 3D cell culture systems. |
| 3D Models and Assays | Creative Bioarray provides a rich resource across a range of cancer types, which then can be cultured into 3D models. |
| 3D Invasion Assay | Creative Bioarray believes that the rapid, automatable 3D invasion system that enables highly reproducible experimental conditions and high throughput analysis will give our customers the best results for identifying novel therapeutic agents. |
Reference
- Fontana F, et al. (2020). "Three-Dimensional Cell Cultures as an In Vitro Tool for Prostate Cancer Modeling and Drug Discovery." Int J Mol Sci. 21 (18), 6806.