In Vitro ADME vs In Vivo ADME
nd in vivo DMPK solutions to support lead optimization, candidate selection, and regulatory submissions, accelerating the path from discovery to clinical development |The biggest differentiators between discovery and development teams are often predictive sciences like ADME. Everyone performs in vitro ADME or in vivo ADME. Unfortunately, some teams consider these experiments interchangeable assets while others regard anything "in vivo" as the proverbial golden ticket (only it usually costs more than gold). In reality each provides different information, has unique strengths and weaknesses, and can inform drug development decisions when utilized properly. Understanding exactly what each can and cannot tell you will save time, money, and help propel your program forward.
In this article we dive into the strengths and weaknesses of in vitro vs in vivo ADME. We will cover what they can (and cannot) reveal about your compound, explain common misconceptions around each technique, and leave you with some tangible next steps you can take to maximize your drug development efforts.
What Is In Vitro ADME and What Can It Tell You?
In vitro ADME methods are laboratory experiments used to predict how a drug will behave in humans. This includes assays performed in cell lines, tissue fractions, or isolated enzymes such as:
- Solubility and chemical stability tests
- Permeability assays using Caco-2 or MDCK cell lines
- Metabolic stability tests in liver microsomes or hepatocytes
- Cytochrome P450 (CYP) inhibition and induction studies
- Plasma protein binding measurements
As you can imagine, in vitro ADME has several huge advantages including speed, control, and throughput. In other words, you can quickly learn about dozens of compounds relative to each other, prioritize hits, and quickly discard red flags before investing more time and money into in vivo studies.
So, what questions can in vitro ADME answer? Here are some of the most common use cases:
- Identifying compounds with high-risk ADME properties early, such as poor solubility, rapid metabolism, or strong efflux.
- Ranking compounds based on relative ADME performance.
- Informing structure-activity relationship (SAR) decisions to guide chemical optimization.
- Providing mechanistic insights into metabolic pathways and transporter interactions.
However, it is equally important to recognize what in vitro studies cannot reliably tell you:
- How the compound behaves in a living system.
- Real pharmacokinetics, including actual plasma concentrations over time.
- Nonlinear kinetics or complex metabolic interactions involving multiple organs.
- Target tissue exposure and distribution patterns in vivo.
Ok, so in vitro ADME can't give you absolute truths. But that doesn't make the data any less useful. In vitro ADME is perfect for making early go, no-go decisions. If your experiments raise red flags it's usually time to go back to the drawing board.
What About In Vivo ADME?
In vivo ADME studies are designed to reveal how a compound behaves in an animal model (and eventually, humans). Common in vivo ADME experiments include:
- Plasma pharmacokinetics (PK): absorption, distribution, metabolism, and elimination over time.
- Bioavailability (F): the fraction of the administered dose that reaches systemic circulation.
- Tissue distribution studies: understanding where the drug actually goes.
- Metabolic pathway identification and elimination mechanisms.
- Species-specific differences: critical for translational studies.
Unlike in vitro ADME, these studies give you a chance to see how your drug behaves in a complex system with many interacting parts. Mechanistically this means the liver, intestine, kidney, and plasma all influence PK. You can finally find out how much drug reaches your target tissue, how long it stays there, and what dosage might be needed to achieve a therapeutic effect.
In vivo ADME is particularly valuable for:
- Determining true systemic exposure (Cmax, AUC, half-life).
- Measuring clearance and volume of distribution under physiological conditions.
- Understanding tissue penetration to ensure the compound reaches the intended target.
- Supporting downstream efficacy and toxicity studies.
As you can imagine in vivo ADME is much more expensive, slow, and difficult to perform high throughput. These studies do not decide between hundreds of compounds like in vitro assays can. In vivo is reserved for late-stage leads where drug-likeness has already been established.
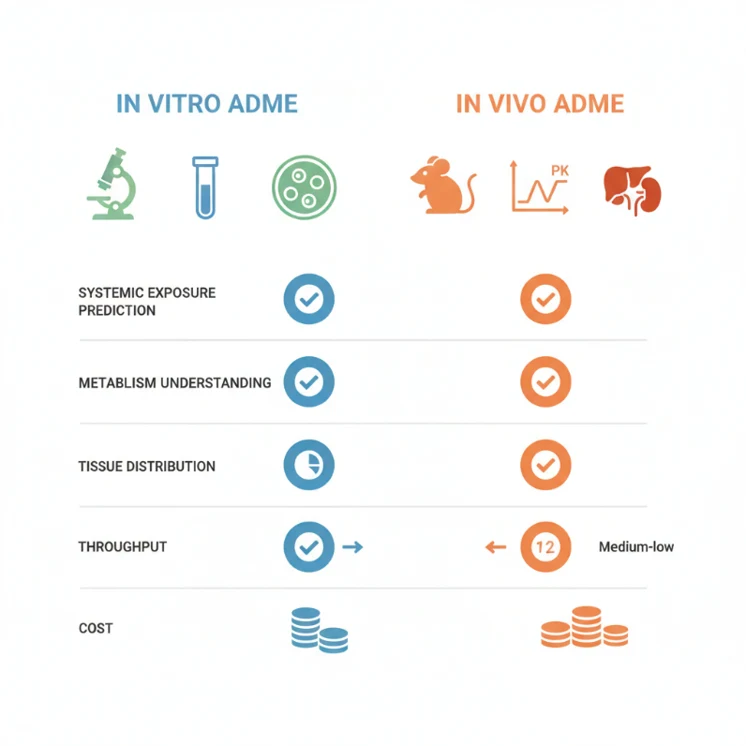
Understanding the strengths and limitations of each approach is essential for designing an efficient ADME strategy. Here's a practical comparison:
| Feature / Question | In Vitro ADME | In Vivo ADME |
|---|---|---|
| Can it predict systemic exposure accurately? | No, only trends or relative predictions | Yes, provides actual PK profiles |
| Can it reveal metabolic pathways? | Partially, mechanisms in isolated systems | Yes, integrates all in vivo metabolism |
| Can it identify tissue distribution? | No | Yes, reveals target and off-target tissue exposure |
| Throughput / speed | High | Low |
| Cost per compound | Low | High |
| Decision-making value | Early risk identification and SAR guidance | Essential for dose selection, efficacy, and safety predictions |
| Limitations | Simplified models, single enzyme/cell bias | Complex, species-specific, resource-intensive |
| Feature / Question | In Vitro ADME | In Vivo ADME |
This comparison clarifies that in vitro ADME is a screening and hypothesis-building tool, while in vivo ADME is a verification and decision-enabling tool.
Common Misconceptions About ADME
Even the most veteran drug teams misuse ADME data from time to time. Let's review some dysfunctional patterns to avoid:
Overreliance on in vitro ADME:
- Assuming microsomal stability directly predicts in vivo clearance.
- Using a single permeability assay to infer oral absorption.
- Believing "all in vitro metrics are acceptable" means a compound is ready for IND submission.
Misunderstanding in vivo ADME:
- Treating in vivo as a final verification step rather than a critical decision point.
- Conducting in vivo studies without a clear hypothesis, leading to inconclusive or misleading data.
These misapplications can result in wasted time, unnecessary costs, and delayed project progression.
Which One Should You Use?
Knowing WHEN to use each technique is just as important as understanding what they can and cannot tell you. To summarize:
You should be using in vitro ADME when:
- Screening hits to leads.
- Lead optimizing in the early phases.
- Rapidly assessing risk across dozens of compounds.
- Learning basic mechanistic details.
You should consider in vivo ADME when:
- Validating candidates for progression.
- Projects are dose-dependent and in vitro is no longer reducing risk.
- "No" is no longer an acceptable decision outcome.
Rules of thumb:
- Use in vitro if you are asking "Does this compound have ANY major risks?"
- Use in vivo if you are asking "What will THIS specific compound do?"
(1) Best Practices: Integrating In Vitro and In Vivo ADME
Teams who master ADME understand in vitro and in vivo experiments are two sides of the same coin. Used incorrectly they can hurt your program and waste your money. Here are some tips to use ADME data to your teams advantage:
- Start with hypotheses.
- Use in vitro data to identify potential risks or generate mechanistic questions.
- Design in vivo experiments to confirm or refute your hypotheses.
- Create feedback loops between in vitro and in vivo: (1) Use in vivo findings to refine in vitro models, improving predictivity for future compounds. (2) Optimize resource allocation and reduce unnecessary experimentation.
(2) Indicators of a mature ADME strategy:
- Fewer experiments, but higher decision value.
- Better integration between early screening and in vivo validation.
- Data that directly informs Go / No-Go decisions.
- Questions Every Drug Developer Should Ask
(3) Before choosing in vitro or in vivo ADME, consider:
- Do we need more data, or more certainty?
- Can continuing in vitro experiments meaningfully change the decision?
- Which ADME approach will best support the next key milestone in our program?
Answering these questions ensures resources are used efficiently, and decisions are based on science, not convenience.
Conclusion
Understanding the strengths and limitations of each tool allowed us to drill down on exactly what in vitro and in vivo ADME can and cannot tell you. Applied properly both techniques will streamline your decision-making process, limit frustrating failures, and accelerate your drug development program.
If your team would like help building an integrated ADME strategy to propel your drug discovery projects forward please don't hesitate to reach out. Whether you are screening dozens of leads or readying a candidate for IND-enabling studies, SDIA can help.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
|---|---|
| Safety Evaluation Services | Creative Bioarray stands as a premier provider of comprehensive safety evaluation studies, adeptly serving a diverse spectrum of industries. Our expertise extends to a broad range of product categories, encompassing pharmaceuticals, cosmetics, and personal care items within the realm of daily chemical products, as well as disinfectants and food products |
| Drug Metabolism and Pharmacokinetics (DMPK) | Creative Bioarray provide comprehensive in vitro a |