Lipophilicity and pKa Assays
- Service Details
- Features
- FAQ
- Explore Other Options

Physicochemical profiling—including assessment of solubility, permeability, lipophilicity, and pKa—is fundamental to early drug discovery success. These properties directly affect bioavailability, clearance, half-life, and overall efficacy, while suboptimal values often lead to poor in vitro predictability, high attrition rates, and costly late-stage failures. Predicting logP/logD and pKa at an early stage gives a clear view on structure–property relationship that is very useful for logical optimization. Employing early decision-making processes improves success probabilities significantly.
What is LogP/LogD and pKa?
- LogP (Partition coefficient): Partition coefficient (P) is the logarithm of the ratio of the concentration of the non-ionized form of a compound in a lipid phase (commonly n-octanol) to that in an aqueous phase.
- LogD (Distribution coefficient): Distribution coefficient (D) refers to the partitioning of the ionized as well as non-ionized form of a molecule at a given pH. The distribution coefficient (LogD) is a more realistic property of the molecule, which determines its ability to cross membranes.
- pKa (Acid dissociation constant): pKa measures a molecule's ability to donate or accept a proton in aqueous solution and can be used to determine the degree of ionization of a molecule at any given pH value. In the case of weak acid and weak base drugs, their degree of ionization in the different parts of the body with different pH (e.g., stomach pH ~1-3, pH of blood ~7.4) is very important as it affects the molecule's solubility, permeability, and target binding.
Our Capabilities
LogP/LogD assays:
Shake-Flask Method— As a classic and widely recognized method, this approach directly calculates the LogP value by measuring compound concentrations in both n-octanol and aqueous phases. This method is highly accurate and reliable, making it the preferred choice for validating critical drug candidates.
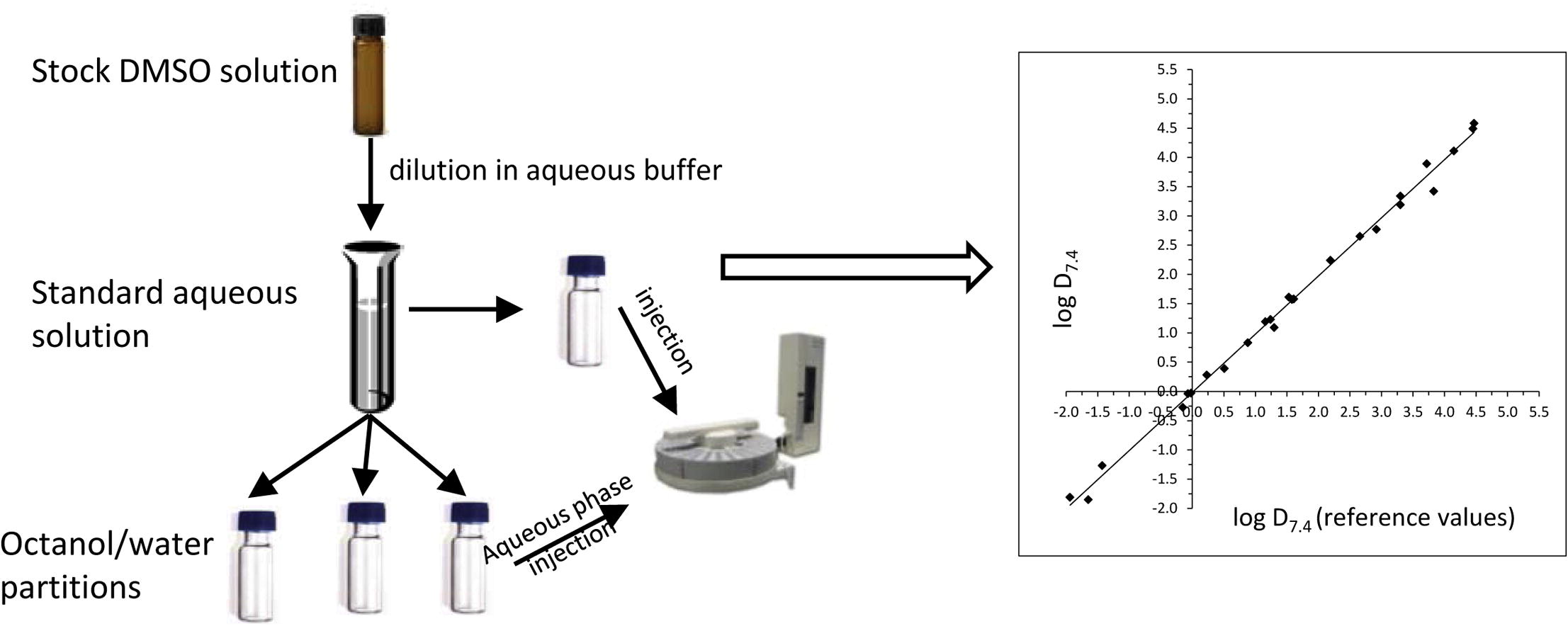 Fig. 1. The determination of the LogP/LogD of drugs by the shake-flask method (Andrés A, Rosés M, et al., 2015).
Fig. 1. The determination of the LogP/LogD of drugs by the shake-flask method (Andrés A, Rosés M, et al., 2015).
HPLC / RP-HPLC Based Methods — This chromatographic approach offers rapid and low-sample estimation of LogP/LogD across diverse compound sets and a broad log range. Suitable when throughput or limited sample mass is a concern.
pH-Metric Titration (automated systems) —This technique provides precise determination of pKa and pH-dependent LogD profiles, including those of multi-pKa compounds. Ideal for poorly soluble or multiprotic molecules.
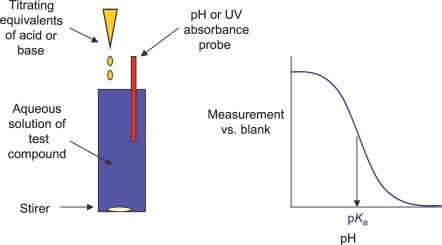 Fig. 2. In-depth potentiometric titration method for pKa determination: pH-metric method (Di L, Kerns E F, et al., 2016).
Fig. 2. In-depth potentiometric titration method for pKa determination: pH-metric method (Di L, Kerns E F, et al., 2016).
pKa assays:
UV-Metric / Spectrophotometric pKₐ — By measuring a compound's UV-Vis absorption spectrum change across a range of pH buffered solutions, its pKa value can be calculated. This method is highly accurate and suitable for molecules containing chromophores.
Capillary Electrophoresis (CE) pKₐ — This technique calculates pKa by measuring a molecule's migration rate in different pH buffers. It requires minimal sample, offers high separation efficiency, and is ideal for small sample volumes.
Deliverables
- Comprehensive reports with LogP/LogD, and pKa values.
- Data analysis and interpretation reports.
Why choose us
Low-sample & difficult chemistries handled
potentiometric, CE, and cosolvent strategies for low-solubility or multiprotic compounds.
Flexible throughput
single-compound to library screening (shake-flask, HPLC or plate-based formats).
Expert interpretation
scientists experienced in DMPK and ADME integration provide actionable context for candidate selection.
Transparent reporting
full metadata, QC metrics and method recommendations for downstream studies.
FAQ
When should I perform Lipophilicity and pKa assessments?
Ideally, you should measure these parameters as early as possible in the drug discovery process, specifically during the lead optimization and candidate selection stages. If you know these values, it will better inform your medicinal chemistry design strategy and guide you to design the right molecules the first time. Late-stage assessment of lipophilicity and pKa that reveals unfavorable ADMET profiles results in costly setbacks for the project.
I only have a tiny amount of sample left. Can you still analyse it?
Absolutely. We offer various micro-analysis methods, such as the Micro-logD method and Capillary Electrophoresis, which are highly effective for precious, limited samples.
How can I interpret the impact of the pKa value on my drug?
The pKa value determines a molecule's ionization state in different bodily pH environments. Generally, a weak acid drug (pKa > 7.4) exists mainly in its non-ionized state in the blood, which facilitates membrane crossing, while a weak base drug (pKa < 7.4) is predominantly ionized. Understanding pKa helps predict a drug's absorption and distribution behavior in the GI tract, blood, and cells.
Do you provide LogP, LogD, and pKₐ in the same study?
Yes. We can package these assays together into a single customised workflow to construct a complete physicochemical profile of your drug candidate.
How does Lipophilicity relate to a drug's solubility?
Lipophilicity and solubility are related but inverse properties. A molecule with high lipophilicity (greasy) will have low aqueous solubility and therefore may be poorly absorbed. On the other hand, a highly hydrophilic (water-loving) molecule will have high solubility but may not cross lipid membranes well. In general, a successful drug candidate will be balanced between these two properties.
References
- Andrés A, Rosés M, et al. Setup and validation of shake-flask procedures for the determination of partition coefficients (logD) from low drug amounts. Eur J Pharm Sci. 2015. 30;76:181-91.
- Di L, Kerns E H. Drug-Like Properties (Second Edition). Academic Press, 2016, 307-312.
Explore Other Options