Oral Tumor Cells
- Background
- Applications
- Scientific Data
Oral tumor cells are a crucial aspect of research in head and neck oncology, reflecting a variety of pathological changes in the tissues of the oral cavity. These cells primarily arise from the squamous epithelium, making oral squamous cell carcinoma (OSCC) the most common malignant tumor in this region. The exploration of oral tumor cells not only underscores the biological mechanisms underpinning their development but also paves the way for innovative therapeutic strategies and diagnostic techniques.
Biological Characteristics of Oral Tumor Cells
- Dysregulated cell cycle. Tumor cells often exhibit uncontrolled proliferation due to mutations in oncogenes and tumor suppressor genes. For instance, alterations in genes like TP53 disrupt cell cycle regulation, leading to increased cellular division and tumor growth.
- Invasive behavior. Many oral tumor cells acquire the ability to invade surrounding tissues, a hallmark of malignancy. This invasive potential is facilitated by changes in cell adhesion molecules and the extracellular matrix.
- Metabolic changes. Cancer cells often undergo metabolic reprogramming, favoring glycolysis over oxidative phosphorylation, a phenomenon known as the Warburg effect. This shift supports rapid growth and survival in a hypoxic environment.
Diagnostic and Prognostic Markers
Oral tumor cells serve as critical tools in the development of diagnostic and prognostic markers. Researchers have identified specific molecular signatures and genetic alterations in oral tumor cells that can predict disease progression and response to treatment. These markers are not only valuable for clinical decision-making but also for the design of personalized therapeutic approaches.
Therapeutic Targets
The investigation of oral tumor cells has led to the identification of novel therapeutic targets. Research has been instrumental in characterizing key signaling pathways and oncogenes that are pivotal to the survival and growth of oral tumor cells. By targeting these pathways, scientists aim to develop new treatments that are more effective and less toxic than traditional modalities.
Immunotherapy and Vaccines
The field of immunotherapy has seen significant advancements, with oral tumor cells playing a central role in the development of novel immunotherapies and vaccines. Researchers are exploring the use of tumor-infiltrating lymphocytes and cancer vaccines to stimulate the immune system to recognize and attack oral tumor cells. These approaches hold the promise of a more durable and less invasive form of treatment for patients with oral cancer.
MiR-205 Induces Cell Apoptosis Through the Activation of Caspase-3/-7 in Human KB Oral Cancer Cells
Miscellaneous studies have examined the biological functions of microRNA-205 (miR-205) as a tumor suppressor in carcinogenesis. the pSuper-miR-205 construct was generated to over-express the miR-205 and transfected into the cultured KB cells. The level of cell viability at 200 ng/ml pSuper-miR-205 was monitored for 72 h. As shown in Fig. 1A (Upper panel), the transfected KB cells with 200 ng/ml pSuper-miR-205 showed approximately lower cell viability by 40% (p < 0.05) compared to the untransfected and pSuper-miR-205 mutation. The up-regulated miR-205 did not alter the cell viability in the NHOKs. Therefore, the viability of the oral KB oral cancer cells was monitored for 72 h after transfection with pSuper-miR-205 according to the dose. As shown in Fig. 1A (Lower panel), up-regulated miR-205 decreased the cell viability in a dose-dependent manner. The KB cells transfected with 200 ng/ml of pSuper-miR-205 exhibited 40% lower cell viability (p < 0.05), as shown in the upper panel data in Fig. 1A. The cell death induced by the up-regulated miR-205 in KB cells was examined further by DAPI staining, which detects the nuclear morphology. As shown in Fig. 1B, the number of cells with the typical morphology of cell death, such as condensed and/or fragmented nuclei, increased under the over-expressed miR-205. Cell death was not detected in either the untransfected or mock cells. The percentage of cell death is presented as a histogram. As shown in Fig. 1B (lower panel), the level of cell death was at least 10-fold higher than either the untransfected or pSupermiR-205 mutation.
To determine if miR-205 has proapoptotic properties, the level of caspase-3/-7 activation was examined as a possible cell death mechanism in KB cells with up-regulated miR-205. As shown in Fig. 1C, the KB cells containing up-regulated miR-205 showed a marked increase in the number of caspase-3/-7-positive apoptotic cells. Moreover, the typical morphology of KB cells was lost and the number of KB cells was decreased significantly by the up-regulated miR-205 compared to the untransfected and pSuper-miR-205mutation. In contrast, neither untransfected nor pSuper-miR-205 mutation activated caspase-3/-7 or altered the cell morphology compared to the KB cells with up-regulated miR-205. Therefore, up-regulated miR-205 induced oral cancer KB cell death through the activation of caspase-3/-7. An immunoblotting assay for the expression of the apoptotic factors, such as PARP and cleaved caspase-3, was performed to confirm the miR-205-induced apoptosis in KB cells. Over-expressed miR-205 significantly cleaved PARP more than the pSuper-miR-205 mutation. In addition, in the pSuper-miR-205 transfected cells, cleaved caspase-3 was expressed significantly more than the pSuper-miR-205 mutation. Therefore, overexpressed miR-205 induces the apoptosis of KB oral cancer cells.
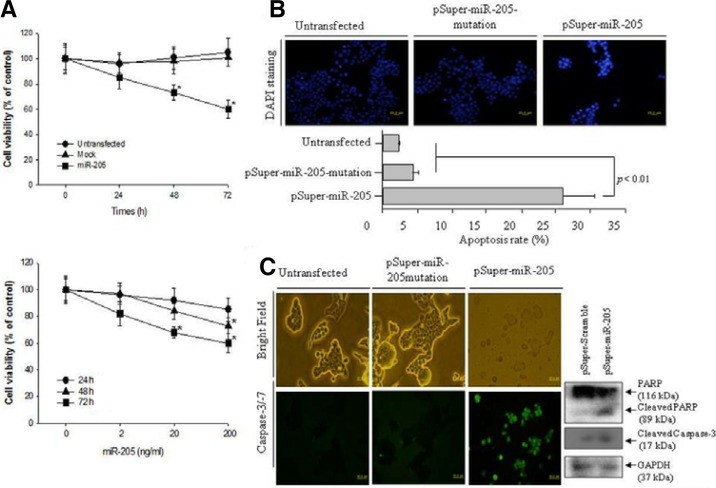 Fig.
1 Over-expressed miR-205 increased the cell cytotoxicity via apoptotic cell death in KB oral cancer cells. (A)
The measurement of cell cytotoxicity by up-regulated miR-205 as time-dependent (upper panel) and dose-dependent
manner (low panel). (B) The observation of apoptotic cell death was performed by DAPI staining after
transfection of 200 ng/ml pSuper-miR-205 and 200 ng/ml pSuper-miR-205mutation. (C) Apoptosis executioner
caspase-3/7 activity was determined using the cell-permeable fluorogenic substrate. (Kim JS, et al,
2012)
Fig.
1 Over-expressed miR-205 increased the cell cytotoxicity via apoptotic cell death in KB oral cancer cells. (A)
The measurement of cell cytotoxicity by up-regulated miR-205 as time-dependent (upper panel) and dose-dependent
manner (low panel). (B) The observation of apoptotic cell death was performed by DAPI staining after
transfection of 200 ng/ml pSuper-miR-205 and 200 ng/ml pSuper-miR-205mutation. (C) Apoptosis executioner
caspase-3/7 activity was determined using the cell-permeable fluorogenic substrate. (Kim JS, et al,
2012)
Changes in Gene Expression in Co-culture of Normal PDL Fibroblasts With SCC-25 Cells
Fibroblast (Fibs) contribution to neoplastic progression, tumor growth, angiogenesis, and metastasis has been recently reported. In the co-culture, fibroblasts were converted to carcinoma-associated fibroblasts (CAFs), which in return initiated epithelial-mesenchymal transition (EMT) of SCC-25 lingual squamous cell carcinoma cells. Normal human periodontal filament fibroblasts (PDLs), and SCC-25 cells were co-cultured for 7 days. After this time gene expression changes in PDLs and SCC-25 cells were investigated, and compared with control cells. The controls received the same cultural conditions, but they were not co-cultured with the partners. By this analysis, in the case of fibroblasts, changes in stroma-derived factor-1 (SDF-1) were investigated in co-culture vs. controls. 1.6-times significant (p < 0.01 by one-way ANOVA) reproducible increase of SDF expression was found in co-cultured fibroblasts compared with control PDL fibroblasts, which indicates the development of carcinoma-associated fibroblasts of PDLs. In the same co-culture system gene expression changes of key genes for EMT were investigated in co-cultured SCC-25 cells vs. control SCC-25 cells. A significant increase in vimentin mRNA expression was detected (Fig. 2A), while E-cadherin mRNA expression was dramatically decreased (Fig. 2B) (p < 0.05). The snail was detected in the control and co-cultured SCC-25 cells; its gene expression increase in co-culture was not statistically significant (p = 0.27) (Fig. 2C). Significant (p < 0.01) correlation was found between gene expression of vimentin and snail (correlation coefficient: 0.95). A significant (p < 0.01) negative correlation was found between the gene expression of vimentin and E-cadherin (correlation coefficient: -0.81), and a statistically significant (p = 0.05) correlation was found between the gene expression of snail and E-cadherin (correlation coefficient: 0.37). These changes represent the characteristic gene expression changes involved in EMT.
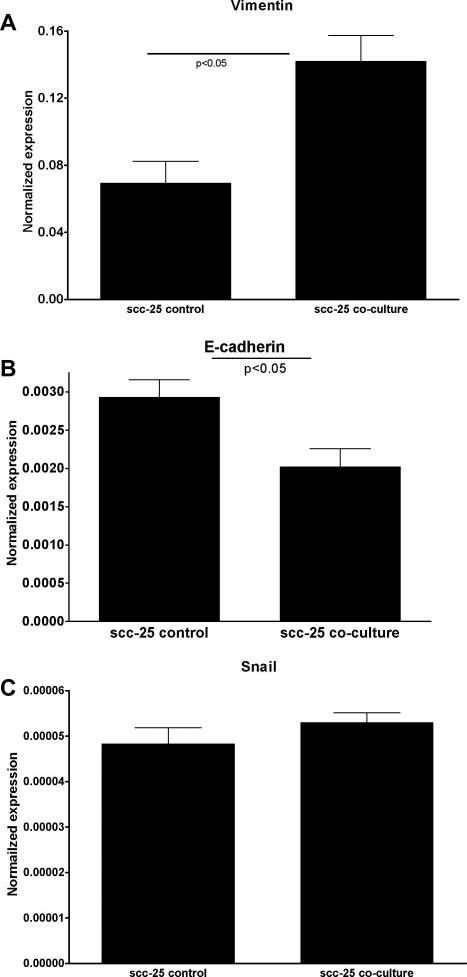 Fig. 2 EMT-related gene expression
changes in co-cultured SCC-25 cells. Key genes for EMT were investigated in co-cultured SCC-25 cells vs. control
SCC-25 cells. A significant increase in vimentin mRNA expression was detected (A), while E-cadherin mRNA
expression was dramatically decreased (B). The snail was detected in the control and co-cultured SCC-25 cells;
its gene expression increase in co-culture was not statistically significant (C). (Dudás J, et al.,
2010)
Fig. 2 EMT-related gene expression
changes in co-cultured SCC-25 cells. Key genes for EMT were investigated in co-cultured SCC-25 cells vs. control
SCC-25 cells. A significant increase in vimentin mRNA expression was detected (A), while E-cadherin mRNA
expression was dramatically decreased (B). The snail was detected in the control and co-cultured SCC-25 cells;
its gene expression increase in co-culture was not statistically significant (C). (Dudás J, et al.,
2010)
Filters Clear all filters
Species
- African clawed frog (1)
- American mink (1)
- Asian tiger mosquito (1)
- Atlantic salmon (1)
- Bluegill (2)
- Bluestriped grunt (1)
- Bovine (7)
- Brazilian free-tailed bat (1)
- Brown bullhead (2)
- Cabbage looper (1)
- Cabbage moth (6)
- Cat (4)
- Central mudminnow (1)
- Chicken (3)
- Chinese hamster (5)
- Chinook salmon (2)
- Chum salmon (1)
- Coho salmon (1)
- Common carp (2)
- Cotton-top tamarin (1)
- Dog (2)
- Fall armyworm (3)
- Fathead minnow (2)
- Fruit fly (1)
- Gilthead sea bream (2)
- Golden hamster (7)
- Goldfish (6)
- Gray dwarf hamster (1)
- Green monkey (2)
- Gypsy moth (1)
- Horse (1)
- Human (998)
- Japanese eel (1)
- Japanese rice fish (7)
- Koi carp (1)
- Mouse (315)
- Mouse x Gray dwarf hamster (1)
- Mouse x Rat (20)
- Northern pike (1)
- Pig (3)
- Rabbit (2)
- Rainbow trout (3)
- Rat (115)
- Rhesus macaque (1)
- Salt marsh moth (1)
- Sheep (2)
- Snakehead murrel (2)
- Sockeye salmon (1)
- Vervet monkey (2)
- Zebrafish (2)
Source
- Abdomen (1)
- Abdomen Metastasis (2)
- Adipose (2)
- Adrenal Gland (8)
- Adrenal Gland Metastasis (2)
- Aorta (4)
- Artery (1)
- Ascites (28)
- Ascites Metastasis (37)
- Bile Duct (3)
- Bladder (25)
- Bladder Metastasis (1)
- Blastocyst (1)
- Blastula (1)
- Blood (127)
- Bone (27)
- Bone Marrow (57)
- Bone Marrow Metastasis (18)
- Bone Metastasis (6)
- Brain (55)
- Brain Metastasis (8)
- Breast (30)
- Bronchus (1)
- Caudal Peduncle (1)
- Caudal Trunk (2)
- Cecum (3)
- Cerebrospinal Fluid (1)
- Cerebrospinal Fluid Metastasis (1)
- Cervix (32)
- Colon (90)
- Connective Tissue (7)
- Cornea (3)
- Cutaneous Metastasis (1)
- Dermis (2)
- Duodenum (1)
- Embryo (29)
- Endometrium (17)
- Esophagus (44)
- Eye (12)
- Eye Socket (5)
- Fetus (3)
- Fin (9)
- Foreskin (4)
- Gallbladder (1)
- Gingiva (2)
- Globe (2)
- Glomerulus (2)
- Groin (1)
- Head Kidney (2)
- Heart (4)
- Hemolymph (1)
- Hypodermis Metastasis (5)
- Ileum (1)
- Intestine (94)
- Jejunum (1)
- kidney (1)
- Kidney (27)
- Liver (35)
- Liver Metastasis (17)
- Lung (58)
- Lung Metastasis (8)
- Lymph Node (7)
- Lymph Node Metastasis (59)
- Muscle (7)
- Muscle Metastasis (2)
- Nose (2)
- Omentum Metastasis (2)
- Oral Cavity (10)
- Ovary (21)
- Ovary Metastasis (2)
- Pancreas (19)
- Pelvic Wall Metastasis (1)
- Pelvis (1)
- Perianal Space Metastasis (1)
- Pericardial Effusion (1)
- Pericardial Effusion Metastasis (2)
- Perineus (1)
- Peripheral Blood (126)
- Peripheral Nervous System (21)
- Peritoneal Effusion (2)
- Peritoneum (1)
- Peritoneum Metastasis (1)
- Pharynx (3)
- Pituitary Gland (7)
- Pleural Effusion (54)
- Pleural Effusion Metastasis (46)
- Prostate (7)
- Rectum (15)
- Renal Pelvis (1)
- Retroperitoneal Space (2)
- Salivary Gland (2)
- Skeletal Muscle (5)
- Skin (32)
- Skin Metastasis (3)
- Small Intestine (4)
- Small Intestine Metastasis (1)
- Smooth Muscle (2)
- Soft Tissue (1)
- Soft Tissue Metastasis (1)
- Spinal Cord (2)
- Stomach (4)
- Testis (15)
- Thoracic Cavity Metastasis (6)
- Thymus (5)
- Thyroid Gland (16)
- Thyroid Gland Metastasis (1)
- Tongue (5)
- Trachea (1)
- Umbilical Cord (1)
- Umbilical Cord Blood (1)
- Urachus (1)
- Ureter (1)
- Uterus (54)
- Uvea (2)
- Vagina (2)
- Vulva (1)
Disease
- Acute Biphenotypic Leukemia (1)
- Acute Erythroid Leukemia (4)
- Acute Megakaryoblastic Leukemia (4)
- Acute Monocytic Leukemia (9)
- Acute Myeloid Leukemia (25)
- Acute Promyelocytic Leukemia (2)
- Adrenal Gland Neuroblastoma (11)
- Adult B Acute Lymphoblastic leukemia (1)
- Adult B Acute Lymphoblastic Leukemia (6)
- Adult T Acute Lymphoblastic Leukemia (6)
- Adult T Lymphoblastic Lymphoma (2)
- Adult T-Cell Leukemia/Lymphoma (1)
- Alveolar Rhabdomyosarcoma (4)
- Alveolar Ridge Squamous Cell Carcinoma (1)
- Amelanotic Melanoma (3)
- Ampulla of Vater Adenocarcinoma (1)
- Ampulla of Vater Adenosquamous Carcinoma (3)
- Anaplastic Astrocytoma (3)
- Anaplastic Large Cell Lymphoma (7)
- Askin Tumor (1)
- Astrocytoma (5)
- B Acute Lymphoblastic Leukemia (2)
- B-Cell Non-Hodgkin Lymphoma (5)
- Bare Lymphocyte Syndrome Type 2 (1)
- Barrett Adenocarcinoma (2)
- Benign Prostatic Hyperplasia (1)
- Bladder Carcinoma (14)
- Bladder Squamous Cell Carcinoma (1)
- Bovine Leukemia (2)
- Breast Adenocarcinoma (4)
- Breast Carcinoma (9)
- Breast Ductal Carcinoma (2)
- Burkitt Lymphoma (17)
- Canavan Disease (1)
- Canine Histiocytic Sarcoma (1)
- Cecum Adenocarcinoma (3)
- Central Nervous System Lymphoma (2)
- Cervical Adenocarcinoma (2)
- Cervical Adenosquamous Carcinoma (2)
- Cervical Small Cell Carcinoma (1)
- Cervical Squamous Cell Carcinoma (2)
- Chicken Bursal Lymphoma (2)
- Childhood B Acute Lymphoblastic Leukemia (13)
- Childhood T Acute Lymphoblastic Leukemia (16)
- Childhood T Lymphoblastic Lymphoma (1)
- Cholangiocarcinoma (2)
- Chronic Eosinophilic Leukemia (1)
- Chronic Lymphocytic Leukemia (2)
- Chronic Myeloid Leukemia (23)
- Clear Cell Renal Cell Carcinoma (2)
- Colon Adenocarcinoma (55)
- Colon Carcinoma (34)
- Colorectal Adenocarcinoma (1)
- Colorectal Carcinoma (1)
- Congenital Pure Red Cell Aplasia (1)
- Cutaneous Melanoma (10)
- Dedifferentiated Chondrosarcoma (1)
- Desmoplastic Melanoma (1)
- Diffuse Large B-Cell Lymphoma (28)
- Down Syndrome (2)
- EBV-Related Burkitt Lymphoma (12)
- Embryonal Carcinoma (3)
- Embryonal Rhabdomyosarcoma (3)
- Endometrial Adenocarcinoma (13)
- Endometrial Adenosquamous Carcinoma (2)
- Endometrial Carcinoma (2)
- Endometrioid Stromal Sarcoma (1)
- Epithelioid Hemangioendothelioma (1)
- Epithelioid Sarcoma (3)
- Esophageal Adenocarcinoma (6)
- Esophageal Squamous Cell Carcinoma (41)
- Essential Thrombocythemia (1)
- Ewing Sarcoma (2)
- Extraskeletal Myxoid Chondrosarcoma (1)
- Fanconi Anemia (1)
- Fibrosarcoma (1)
- Follicular Lymphoma (2)
- Gallbladder Carcinoma (2)
- Gallbladder Undifferentiated Carcinoma (2)
- Gastric Adenocarcinoma (6)
- Gastric Adenosquamous Carcinoma (1)
- Gastric Carcinoma (5)
- Gastric Choriocarcinoma (1)
- Gastric Fundus Carcinoma (1)
- Gastric Signet Ring Cell Adenocarcinoma (1)
- Gastric Small Cell Carcinoma (2)
- Gastric Tubular Adenocarcinoma (5)
- Gastroesophageal Junction Adenocarcinoma (1)
- Gestational Choriocarcinoma (1)
- Gingival Squamous Cell Carcinoma (2)
- Glioblastoma (18)
- Gliosarcoma (1)
- Goldfish Erythrophoroma (4)
- Hairy Cell Leukemia (1)
- Hamster Kidney Tumor (1)
- Hamster Pancreatic Ductal Adenocarcinoma (1)
- Hamster Uterine Leiomyosarcoma (1)
- Hepatoblastoma (2)
- Hepatocellular Carcinoma (6)
- Hepatosplenic T-Cell Lymphoma (2)
- Hereditary Thyroid Gland Medullary Carcinoma (1)
- High Grade B-Cell Lymphoma (1)
- High Grade Ovarian Serous Adenocarcinoma (8)
- Hodgkin Lymphoma (9)
- Hypopharyngeal Squamous Cell Carcinoma (2)
- Infectious Mononucleosis (1)
- Intrahepatic Cholangiocarcinoma (6)
- Invasive Breast Carcinoma of No Special Type (12)
- Invasive Breast Lobular Carcinoma (1)
- Kidney Neoplasm (1)
- Kidney Rhabdoid Tumor (1)
- Krukenberg Tumor (1)
- Liposarcoma (1)
- Lung Adenocarcinoma (17)
- Lung Giant Cell Carcinoma (8)
- Lung Large Cell Carcinoma (9)
- Lung Mucoepidermoid Carcinoma (1)
- Lung Non-Small Cell Carcinoma (2)
- Lung Small Cell Carcinoma (25)
- Lung Squamous Cell Carcinoma (9)
- Lymphoblastic Lymphoma (1)
- Malignant Peripheral Nerve Sheath Tumor (1)
- Mantle Cell Lymphoma (5)
- Mature Gastric Teratoma (1)
- Maxillary Sinus Squamous Cell Carcinoma (1)
- Medaka Hepatoma (2)
- Medulloblastoma (3)
- Melanoma (24)
- Meningioma (2)
- Minimally Invasive Lung Adenocarcinoma (1)
- Monophasic Synovial Sarcoma (1)
- Mouse Bladder Transitional Cell Carcinoma (1)
- Mouse Chondrosarcoma (1)
- Mouse Colon Adenocarcinoma (3)
- Mouse Ependymoma (2)
- Mouse Erythroid Leukemia (13)
- Mouse Fibrosarcoma (5)
- Mouse Glioblastoma (1)
- Mouse Hemangioendothelioma (1)
- Mouse Hepatocellular Carcinoma (1)
- Mouse Insulinoma (3)
- Mouse Intestinal Tract Neuroendocrine Adenoma (1)
- Mouse Islet Cell Adenoma (1)
- Mouse Kidney Carcinoma (1)
- Mouse Leukemia (10)
- Mouse Leydig Cell Tumor (1)
- Mouse Lymphoma (8)
- Mouse Mammary Gland Malignant Neoplasm (23)
- Mouse Melanoma (9)
- Mouse Multiple Myeloma (5)
- Mouse Myeloid Leukemia (3)
- Mouse Neoplasm (1)
- Mouse Neuroblastoma (21)
- Mouse Oral Cavity Squamous Cell Carcinoma (1)
- Mouse Osteosarcoma (3)
- Mouse Pituitary Gland Neoplasm (1)
- Mouse Plasmacytoma (1)
- Mouse Precursor T Cell Lymphoblastic Lymphoma/Leukemia (2)
- Mouse Pulmonary Adenoma (1)
- Mouse Pulmonary Malignant Tumor (3)
- Mouse Pulmonary Squamous Cell Carcinoma (1)
- Mouse Rectum Carcinoma (2)
- Mouse Reticulum Cell Sarcoma (2)
- Mouse Sarcoma (1)
- Mouse Teratocarcinoma (8)
- Mouse Thymic Lymphoma (3)
- Mycosis Fungoides (1)
- Myelodysplastic Syndrome (1)
- Myxofibrosarcoma (1)
- Natural Killer Cell Lymphoblastic Leukemia/Lymphoma (2)
- Neuroblastoma (26)
- Oral Cavity Squamous Cell Carcinoma (15)
- Osteoid Osteoma (1)
- Osteosarcoma (15)
- Ovarian Carcinoma (1)
- Ovarian Clear Cell Adenocarcinoma (1)
- Ovarian Endometrioid Adenocarcinoma (4)
- Ovarian Granulosa Cell Tumor (1)
- Ovarian Mucinous Adenocarcinoma (2)
- Ovarian Serous Adenocarcinoma (2)
- Ovarian Serous Cystadenocarcinoma (2)
- Ovarian Small Cell Carcinoma (1)
- Pancreatic Adenocarcinoma (13)
- Pancreatic Carcinoma (5)
- Pancreatic Ductal Adenocarcinoma (12)
- Papillomavirus-Independent Cervical Squamous Cell Carcinoma (1)
- Papillomavirus-Related Cervical Adenocarcinoma (7)
- Papillomavirus-Related Cervical Squamous Cell Carcinoma (4)
- Papillomavirus-Related Endocervical Adenocarcinoma (16)
- Paroxysmal Nocturnal Hemoglobinuria (3)
- Pharyngeal Squamous Cell Carcinoma (1)
- Plasma Cell Myeloma (15)
- Pleural Epithelioid Mesothelioma (5)
- Pleural Sarcomatoid Mesothelioma (2)
- Poorly Differentiated Thyroid Gland Carcinoma (1)
- Primary Cutaneous T-Cell Non-Hodgkin Lymphoma (1)
- Primary Effusion Lymphoma (7)
- Primitive Neuroectodermal Tumor (1)
- Prostate carcinoma (1)
- Prostate Carcinoma (9)
- Rat C-Cell Carcinoma (1)
- Rat Cholangiocarcinoma (1)
- Rat Colon Adenocarcinoma (5)
- Rat Digestive System Neoplasm (1)
- Rat Fibrosarcoma (1)
- Rat Hepatocellular Carcinoma (20)
- Rat Histiocytic Sarcoma (1)
- Rat Insulinoma (2)
- Rat Leukemia (1)
- Rat Leydig Cell Adenoma (1)
- Rat Lung Carcinoma (1)
- Rat Malignant Glioma (4)
- Rat Malignant Meningioma (1)
- Rat Malignant Oligodendroglioma (2)
- Rat Malignant Thymoma (3)
- Rat Mammary Gland Adenocarcinoma (10)
- Rat Neuroblastoma (3)
- Rat Osteosarcoma (2)
- Rat Pituitary Gland Neoplasm (6)
- Rat Prostate Adenocarcinoma (3)
- Rat Rhabdomyosarcoma (1)
- Rat Sarcoma (2)
- Rat Squamous Cell Carcinoma (1)
- Rat Urinary Bladder Transitional Cell Carcinoma (2)
- Rat Urinary System Neoplasm (6)
- Rectal Adenocarcinoma (13)
- Rectosigmoid Adenocarcinoma (1)
- Recurrent Bladder Carcinoma (1)
- Renal Cell Carcinoma (7)
- Renal Pelvis Urothelial Carcinoma (1)
- Retinoblastoma (11)
- Sacral Chordoma (1)
- Sacrococcygeal Teratoma (1)
- Salivary Gland Squamous Cell Carcinoma (1)
- Sezary Syndrome (1)
- Shwachman-Diamond Syndrome (1)
- Skin Squamous Cell Carcinoma (2)
- Splenic Marginal Zone Lymphoma (1)
- Testicular Embryonal Carcinoma (8)
- Testicular Teratoma (2)
- Testicular Yolk Sac Tumor (1)
- Thyroid Gland Anaplastic Carcinoma (10)
- Thyroid Gland Follicular Carcinoma (4)
- Thyroid Gland Papillary Carcinoma (3)
- Thyroid Gland Sarcoma (1)
- Thyroid Gland Squamous Cell Carcinoma (2)
- Tongue Adenosquamous Carcinoma (1)
- Tongue Squamous Cell Carcinoma (6)
- Type I Endometrial Adenocarcinoma (1)
- Ureter Urothelial Carcinoma (1)
- Uterine Carcinosarcoma (2)
- Uterine Corpus Leiomyosarcoma (1)
- Uterine Corpus Sarcoma (2)
- Uveal Melanoma (2)
- Vaginal Melanoma (2)
- Vulvar Melanoma (1)
- Vulvar Squamous Cell Carcinoma (1)
Description: Established from the tumor of a 52-year-old Japanese man with highly differentiated squamous cell ...
Description: Established from the tumor of a 60-year-old Japanese man with moderately differentiated squamous ...
Description: The CAL-27 cells are established from the poorly differentiated squamous cell carcinoma of the ...
Description: Established from the surgically removed fragment of a tongue lesion from a 69-year-old man with ...
Description: Human cell line derived from squamous cell carcinoma at mouth floor.
Description: Human cell line derived from tongue cancer. Squamous cell carcinoma.
Description: Human tongue squamous cell carcinoma cell line. HLA-A 2/24.
Description: Human gingival carcinoma cell line. Expressing remarkable EGF receptor. HLA-A 2/24.
Description: Human tongue squamous cell carcinoma derived cell line.
Description: Human cell line derived from oral squamous cell carcinoma occurred in 69-yo, male patient. HLA-A ...
Description: Human cell line derived from metastasis of cancer occurred in oral cavity, the same patient as ...
Description: Human cell line derived from metastasis of cancer occurred in oral cavity, the same patient as ...
Description: Human cell line derived from metastasis of cancer occurred in oral cavity, the same patient as ...
Description: Human cell line derived from metastasis of cancer occurred in oral cavity, the same patient as ...
Description: Human cell line derived from metastasis of cancer occurred in oral cavity, the same patient as ...
Description: Human cell line derived from metastasis of cancer occurred in oral cavity, the same patient as ...
Description: G-CSF and IL-1 producing oral squamous cell carcinoma, the same patient as T3M-1 Clone2 and CJM.
Description: G-CSF producing oral squamous cell carcinoma, the same patient as T3M-1 Cl-10 and CJM. Cell growth ...