Testicular Tumor Cells
- Background
- Applications
- Scientific Data
Testicular tumors are a relatively rare yet significant subset of cancers that predominantly affect young men. Testicular tumors can arise from different cell types within the testes, including germ cells, which give rise to sperm, and non-germ cells, which comprise the supportive tissue. The diversity of cell origins contributes to the varied nature of testicular tumors and their distinct pathologies.
Testicular Tumor Cell Characteristics
- Cellular composition and types. Testicular tumors primarily originate from germ cells, which are responsible for sperm production. The two main categories of germ cell tumors are seminomas and non-seminomas. Seminomas are characterized by their slow growth and better prognosis, while non-seminomas, which include embryonal carcinoma, yolk sac tumors, and choriocarcinoma, are generally more aggressive.
- Genetic and molecular features. Testicular tumor cells exhibit distinct genetic alterations that contribute to their malignancy. Common chromosomal abnormalities include the isochromosome 12p, which is found in approximately 80% of germ cell tumors. This genetic hallmark can serve as a biomarker for diagnosis and monitoring. Additionally, mutations in genes such as KIT and TP53 have been implicated in tumor progression, highlighting potential targets for therapeutic intervention.
- Tumor microenvironment. The tumor microenvironment plays a critical role in the behavior of testicular tumor cells. Interactions between tumor cells and surrounding stromal cells can influence tumor growth and metastasis. For instance, the presence of certain immune cells, such as macrophages, can either promote or inhibit tumor progression. Understanding these dynamics is essential for developing immunotherapeutic strategies.
Drug Screening
Researchers use testicular tumor cell lines and patient-derived xenografts to screen potential anti-cancer drugs. These models allow for the evaluation of drug efficacy and the identification of potential side effects before moving to more complex and costly clinical trials.
Targeted Therapies
The genetic and molecular profiling of testicular tumor cells has led to the identification of specific targets for targeted therapies. For instance, mutations in the KIT gene are associated with certain testicular cancers, and drugs that inhibit KIT activity have shown promise in treating these tumors.
Immunotherapy Research
The field of immunotherapy has benefited from the study of testicular tumor cells. These cells can be used to develop and test immunotherapeutic strategies that harness the patient's immune system to recognize and attack tumor cells.
Cancer Vaccine Research
Research into cancer vaccines often involves the use of testicular tumor antigens to stimulate an immune response. By exposing the immune system to these antigens, it may be possible to train it to target and destroy tumor cells throughout the body.
Autophagy Levels in Cisplatin-Resistant Testicular Cancer Cells Depend on Panx-1 Expression
Pannexin1 (Panx-1) is a gap junction channel protein that mediates the release of intracellular ATP during autophagy and thus plays an important role in tumor cell apoptosis and chemo-resistance. Cisplatin-resistant I-10 testicular cancer cell lines (I-10/CDDP) autophagy-associated proteins (p62, p-mTOR, mTOR, and LC3) exhibited high levels of autophagy in their expression, while LC3-II expression was more significantly in the presence of lysosomal degradation blocked by chloroquine (CQ).
To further explore the Panx-1-induced autophagy in I-10/DDP cells, two independent shRNAs and an expression vector encoding mouse full-length Panx-1 (mPanx-1) were transfected into I-10/DDP cells. The role of the Panx-1 channel on the level of autophagy was further elucidated via shRNA-mediated knockdown and full-length panx-1 gene overexpression. As shown in Fig. 1a, Panx-1 expression was decreased in the shRNA-transfected group compared to the NC group. In addition, Panx-1 knockdown significantly reduced extracellular ATP levels in I-10/CDDP cells (Fig. 1b). Panx-1 knockdown significantly elevated LC3II expression and also significantly decreased p62 and p-mTOR expression in I-10/DDP cells, while the presence of CQ more significantly upregulated LC3II protein expression (Fig. 1c). Furthermore, the autophagy flux of GFP-LC3B and autophagosomes were increased in the Panx-1 knockdown cells (Fig. 1d, e).
Consistent with the above, ectopic expression of Panx-1 (Fig. 2a) increased extracellular ATP levels in I-10/CDDP cells (Fig. 2b). Similarly, ectopic expression of Panx-1 significantly decreased the expression of LC3-II and increased the expression of p62 and p-mTOR, while the presence of CQ more significantly downregulated LC3II protein expression (Fig. 2c). Furthermore, the autophagy flux of GFP-LC3B and autophagosomes was also decreased in the mPanx-1 group (Fig. 2d, e). Taken together, autophagy of I-10/CDDP cells is regulated by the expression of Panx-1.
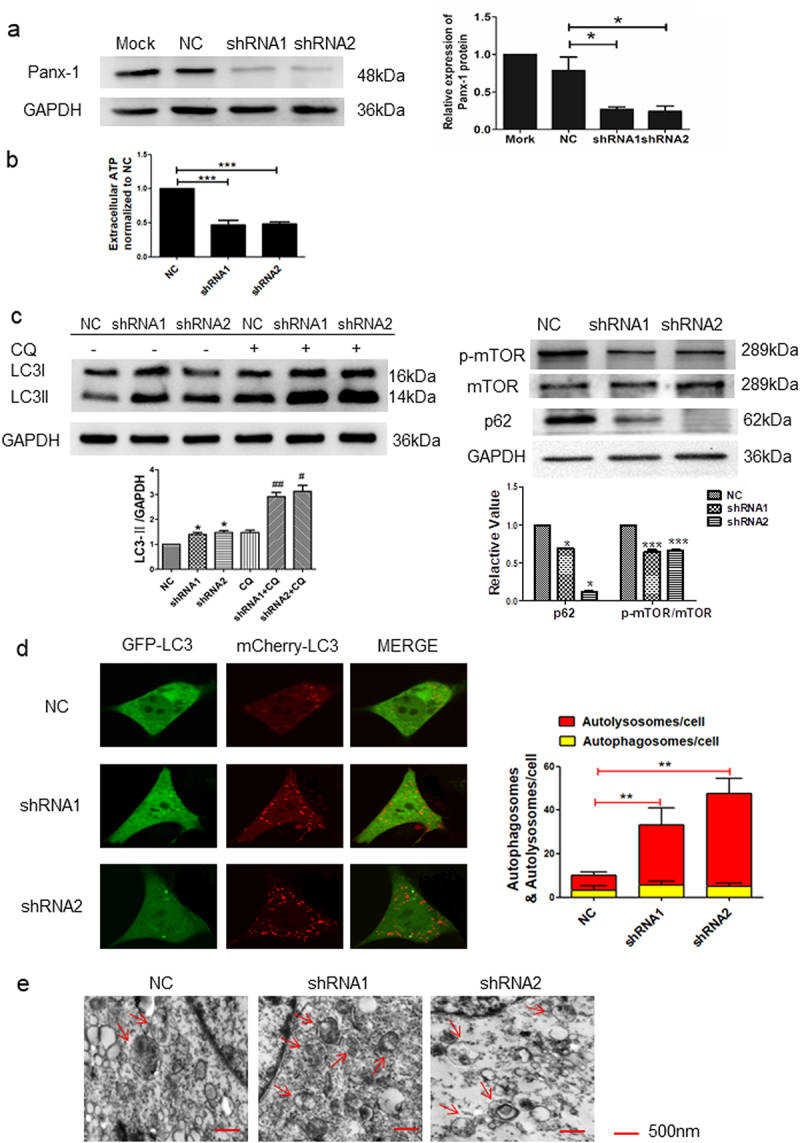 Fig. 1 Knockdown of Panx-1 increased
autophagy in I-10/CDDP cells. (a) Immunoblots showing Panx-1 expression in Mock, NC, and shRNA-Panx-1 I-10/CDDP
cells. (b) The extracellular ATP was assessed by Luminescence assay. (c) Western blots showed the expression of
p62, p-mTOR, and mTOR proteins after the knockdown of Panx-1 and the expression of LC3 protein in the presence
of CQ (10 μmol). (d) Representative fluorescence images showed autophagosomes and autolysosomes in the indicated
group. (e) Representative TEM images showed autophagosomes. (Yuan M, et al, 2022)
Fig. 1 Knockdown of Panx-1 increased
autophagy in I-10/CDDP cells. (a) Immunoblots showing Panx-1 expression in Mock, NC, and shRNA-Panx-1 I-10/CDDP
cells. (b) The extracellular ATP was assessed by Luminescence assay. (c) Western blots showed the expression of
p62, p-mTOR, and mTOR proteins after the knockdown of Panx-1 and the expression of LC3 protein in the presence
of CQ (10 μmol). (d) Representative fluorescence images showed autophagosomes and autolysosomes in the indicated
group. (e) Representative TEM images showed autophagosomes. (Yuan M, et al, 2022)
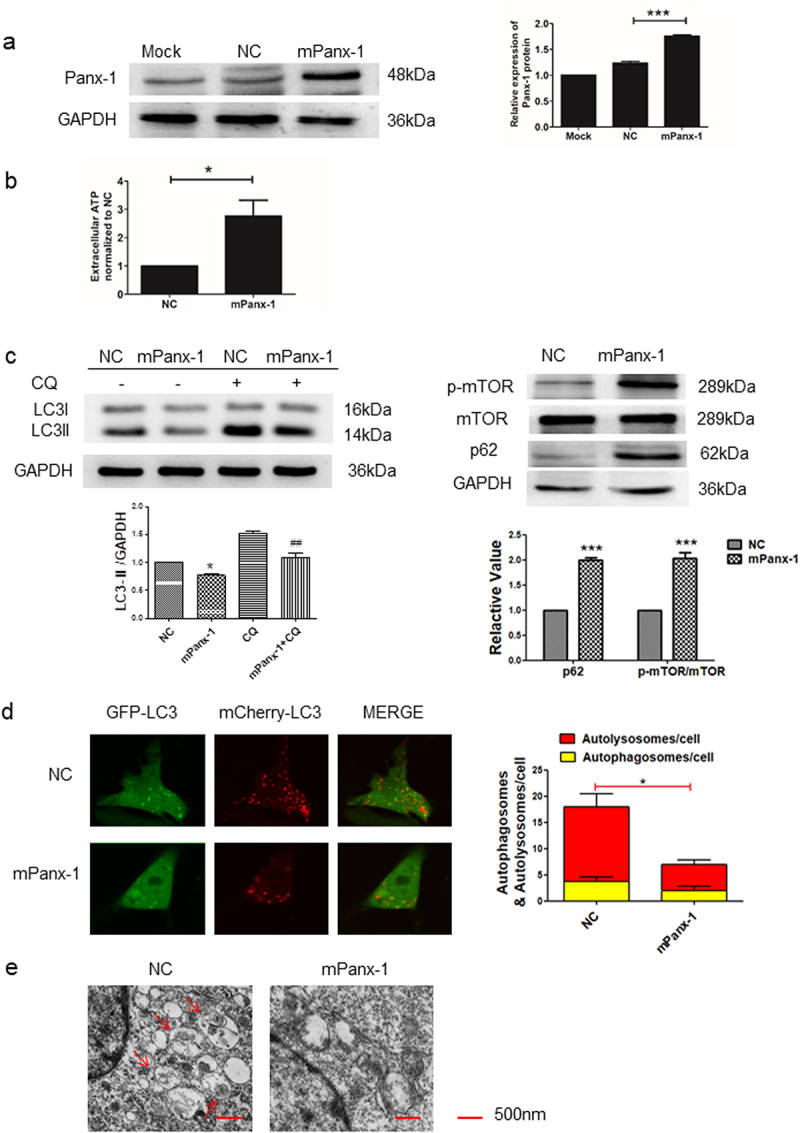 Fig. 2 Overexpression of Panx-1
decreased autophagy in I-10/CDDP cells. (a) Immunoblots showing Panx-1 expression in Mork, NC and mPanx-1
I-10/CDDP cells. (b) The extracellular ATP was assessed by luminescence assay. (c) Western blots showed the
expression of p62, p-mTOR, and mTOR proteins after Panx-1 overexpression and the expression of LC3 protein in
the presence of CQ (10 μmol). (d) Representative fluorescence images showed autophagosomes and autolysosomes in
the indicated group. (e) Representative TEM images showed autophagosomes. (Yuan M, et al, 2022)
Fig. 2 Overexpression of Panx-1
decreased autophagy in I-10/CDDP cells. (a) Immunoblots showing Panx-1 expression in Mork, NC and mPanx-1
I-10/CDDP cells. (b) The extracellular ATP was assessed by luminescence assay. (c) Western blots showed the
expression of p62, p-mTOR, and mTOR proteins after Panx-1 overexpression and the expression of LC3 protein in
the presence of CQ (10 μmol). (d) Representative fluorescence images showed autophagosomes and autolysosomes in
the indicated group. (e) Representative TEM images showed autophagosomes. (Yuan M, et al, 2022)
Cordycepin-Induced Apoptosis in Mouse Testicular Tumor Cells by Activating PERK/EIF2α Signaling Pathways
Studies have demonstrated that misfolded proteins could prompt ER stress to restore protein homeostasis. If stress is prolonged, apoptotic cell death succeeds. To further study whether cordycepin would regulate ER stress pathways inducing apoptosis in the mouse Leydig tumor (MA-10) cells, ER stress-related proteins, such as PERK, EIF2α, p-EIF2α, ATF3, CHOP, ATF6β, IRE1α, cleaved XBP1, and cleaved caspase-12, in cordycepin-treated MA-10 cells were analyzed by Western blotting (Fig. 3A). Results showed that expression of PERK gradually decreased by cordycepin treatments (0-100 μmol/L); however, expression of PERK rebounded by treatments of 500 and 1000 μmol/L cordycepin for 12 hours (Fig. 3B). Interestingly, expression of PERK significantly decreased by cordycepin (50-1000 μmol/L) in 24-hour treatment (Fig. 3B). Expressions of p-EIF2α were stimulated by 12- and 24-hour treatments of 500 and 1000 μmol/L cordycepin, respectively (Fig. 3B). Expression of ATF3 was stimulated by 100 μmol/L cordycepin after 12- and 24-hour treatments (Fig. 3B). The CHOP expression showed no significant change with cordycepin treatments for 12 and 24 hours (Fig. 3B).
Total ATF6β, IRE1α, cleaved XBP1plus total, and cleaved caspase-12 were also detected by Western blotting with the treatments of cordycepin (0, 10, 50, 100, 500, and 1000 μmol/L) for 12 and 24 hours. Results showed that 12- and 24-hour cordycepin treatments did not affect the expressions of ATF6β (Fig. 4A, B). Treatments with cordycepin (10-1000 μmol/L) for 12 and 24 hours significantly decreased the expressions of IRE1α (Fig. 4B). A known value of 1000 μmol/L cordycepin induced the maximal levels of XBP1 expression at 12 hours (Fig. 4B). Furthermore, 50 and 100 μmol/L cordycepin induced the expressions of cleaved caspase-12 at 12 hours (Fig. 4B). These results indicated that cordycepin could regulate ER stress pathways to induce apoptosis in MA-10 cells.
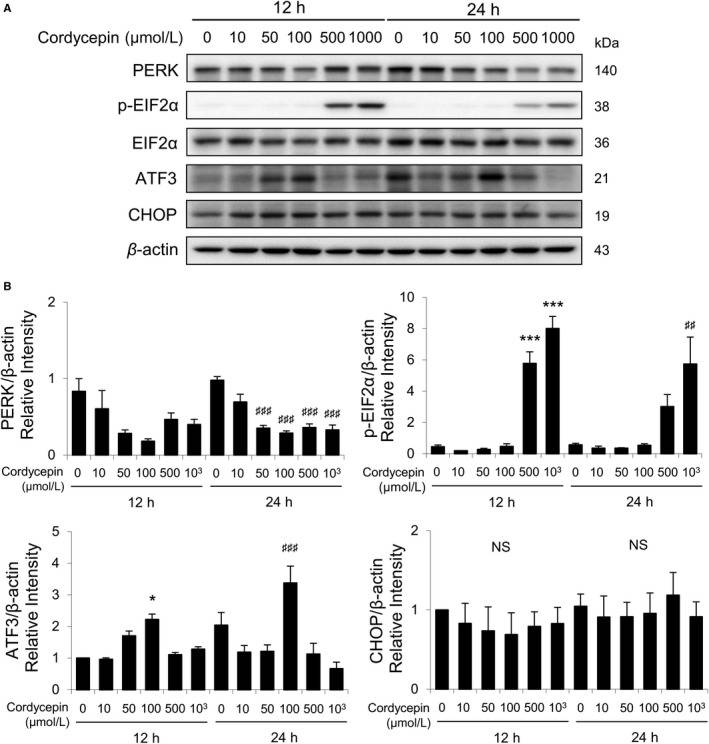 Fig. 3
Cordycepin-induced apoptosis of MA-10 cells by activating PERK/EIF2α signaling pathways. A, Western blot
analysis for the expression of total and phosphorylated EIF2α, total PERK, ATF3, and CHOP in MA-10 cells treated
with 0, 10, 50, 100, 500, or 1000 μmol/L cordycepin for 12 or 24 h, respectively. B, Quantification of bar
graphs shows the IOD of EIF2α, phosphor-EIF2α, PERK, ATF3, and CHOP, which were normalized with β-actin (43 kDa)
in each lane, respectively. (Chang MM, et al., 2019)
Fig. 3
Cordycepin-induced apoptosis of MA-10 cells by activating PERK/EIF2α signaling pathways. A, Western blot
analysis for the expression of total and phosphorylated EIF2α, total PERK, ATF3, and CHOP in MA-10 cells treated
with 0, 10, 50, 100, 500, or 1000 μmol/L cordycepin for 12 or 24 h, respectively. B, Quantification of bar
graphs shows the IOD of EIF2α, phosphor-EIF2α, PERK, ATF3, and CHOP, which were normalized with β-actin (43 kDa)
in each lane, respectively. (Chang MM, et al., 2019)
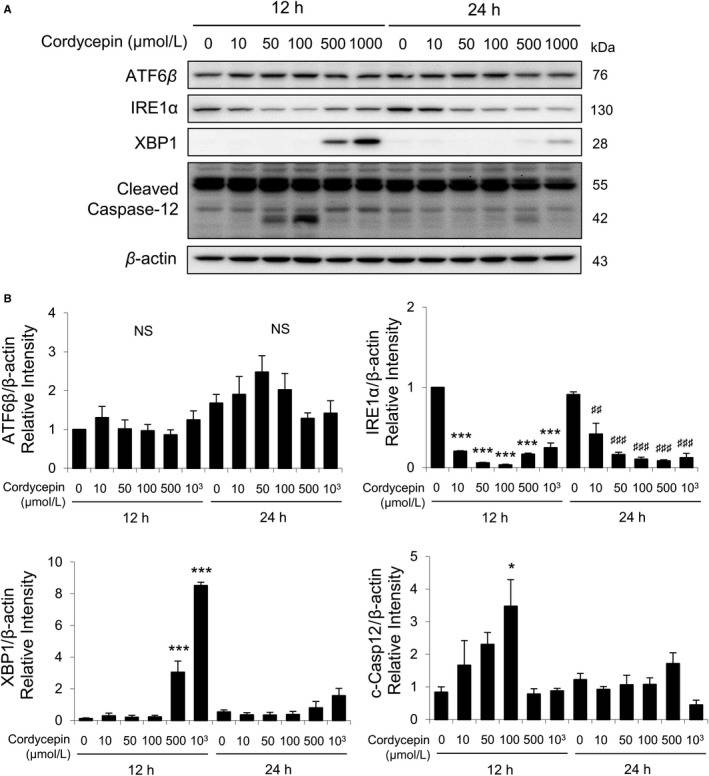 Fig. 4
Cordycepin-induced apoptosis of MA-10 cells by activating ATF6 and IRE1 signaling pathways. A, Western blot
analysis for the expression of total ATF6β, IRE1α, XBP1, total, and cleaved caspase-12 in MA-10 cells treated
with 0, 10, 50, 100, 500, or 1000 μmol/L cordycepin for 12 or 24 h, respectively. B, Quantification of bar
graphs show that the IOD of ATF6β, IRE1α, XBP1, and cleaved caspase-12 (c-Casp12), which were normalized with
β-actin (43 kDa) in each lane, respectively. (Chang MM, et al., 2019)
Fig. 4
Cordycepin-induced apoptosis of MA-10 cells by activating ATF6 and IRE1 signaling pathways. A, Western blot
analysis for the expression of total ATF6β, IRE1α, XBP1, total, and cleaved caspase-12 in MA-10 cells treated
with 0, 10, 50, 100, 500, or 1000 μmol/L cordycepin for 12 or 24 h, respectively. B, Quantification of bar
graphs show that the IOD of ATF6β, IRE1α, XBP1, and cleaved caspase-12 (c-Casp12), which were normalized with
β-actin (43 kDa) in each lane, respectively. (Chang MM, et al., 2019)
Filters Clear all filters
Species
- African clawed frog (1)
- American mink (1)
- Asian tiger mosquito (1)
- Atlantic salmon (1)
- Bluegill (2)
- Bluestriped grunt (1)
- Bovine (7)
- Brazilian free-tailed bat (1)
- Brown bullhead (2)
- Cabbage looper (1)
- Cabbage moth (6)
- Cat (4)
- Central mudminnow (1)
- Chicken (3)
- Chinese hamster (5)
- Chinook salmon (2)
- Chum salmon (1)
- Coho salmon (1)
- Common carp (2)
- Cotton-top tamarin (1)
- Dog (2)
- Fall armyworm (3)
- Fathead minnow (2)
- Fruit fly (1)
- Gilthead sea bream (2)
- Golden hamster (7)
- Goldfish (6)
- Gray dwarf hamster (1)
- Green monkey (2)
- Gypsy moth (1)
- Horse (1)
- Human (998)
- Japanese eel (1)
- Japanese rice fish (7)
- Koi carp (1)
- Mouse (315)
- Mouse x Gray dwarf hamster (1)
- Mouse x Rat (20)
- Northern pike (1)
- Pig (3)
- Rabbit (2)
- Rainbow trout (3)
- Rat (115)
- Rhesus macaque (1)
- Salt marsh moth (1)
- Sheep (2)
- Snakehead murrel (2)
- Sockeye salmon (1)
- Vervet monkey (2)
- Zebrafish (2)
Source
- Abdomen (1)
- Abdomen Metastasis (2)
- Adipose (2)
- Adrenal Gland (8)
- Adrenal Gland Metastasis (2)
- Aorta (4)
- Artery (1)
- Ascites (28)
- Ascites Metastasis (37)
- Bile Duct (3)
- Bladder (25)
- Bladder Metastasis (1)
- Blastocyst (1)
- Blastula (1)
- Blood (127)
- Bone (27)
- Bone Marrow (57)
- Bone Marrow Metastasis (18)
- Bone Metastasis (6)
- Brain (55)
- Brain Metastasis (8)
- Breast (30)
- Bronchus (1)
- Caudal Peduncle (1)
- Caudal Trunk (2)
- Cecum (3)
- Cerebrospinal Fluid (1)
- Cerebrospinal Fluid Metastasis (1)
- Cervix (32)
- Colon (90)
- Connective Tissue (7)
- Cornea (3)
- Cutaneous Metastasis (1)
- Dermis (2)
- Duodenum (1)
- Embryo (29)
- Endometrium (17)
- Esophagus (44)
- Eye (12)
- Eye Socket (5)
- Fetus (3)
- Fin (9)
- Foreskin (4)
- Gallbladder (1)
- Gingiva (2)
- Globe (2)
- Glomerulus (2)
- Groin (1)
- Head Kidney (2)
- Heart (4)
- Hemolymph (1)
- Hypodermis Metastasis (5)
- Ileum (1)
- Intestine (94)
- Jejunum (1)
- kidney (1)
- Kidney (27)
- Liver (35)
- Liver Metastasis (17)
- Lung (58)
- Lung Metastasis (8)
- Lymph Node (7)
- Lymph Node Metastasis (59)
- Muscle (7)
- Muscle Metastasis (2)
- Nose (2)
- Omentum Metastasis (2)
- Oral Cavity (10)
- Ovary (21)
- Ovary Metastasis (2)
- Pancreas (19)
- Pelvic Wall Metastasis (1)
- Pelvis (1)
- Perianal Space Metastasis (1)
- Pericardial Effusion (1)
- Pericardial Effusion Metastasis (2)
- Perineus (1)
- Peripheral Blood (126)
- Peripheral Nervous System (21)
- Peritoneal Effusion (2)
- Peritoneum (1)
- Peritoneum Metastasis (1)
- Pharynx (3)
- Pituitary Gland (7)
- Pleural Effusion (54)
- Pleural Effusion Metastasis (46)
- Prostate (7)
- Rectum (15)
- Renal Pelvis (1)
- Retroperitoneal Space (2)
- Salivary Gland (2)
- Skeletal Muscle (5)
- Skin (32)
- Skin Metastasis (3)
- Small Intestine (4)
- Small Intestine Metastasis (1)
- Smooth Muscle (2)
- Soft Tissue (1)
- Soft Tissue Metastasis (1)
- Spinal Cord (2)
- Stomach (4)
- Testis (15)
- Thoracic Cavity Metastasis (6)
- Thymus (5)
- Thyroid Gland (16)
- Thyroid Gland Metastasis (1)
- Tongue (5)
- Trachea (1)
- Umbilical Cord (1)
- Umbilical Cord Blood (1)
- Urachus (1)
- Ureter (1)
- Uterus (54)
- Uvea (2)
- Vagina (2)
- Vulva (1)
Disease
- Acute Biphenotypic Leukemia (1)
- Acute Erythroid Leukemia (4)
- Acute Megakaryoblastic Leukemia (4)
- Acute Monocytic Leukemia (9)
- Acute Myeloid Leukemia (25)
- Acute Promyelocytic Leukemia (2)
- Adrenal Gland Neuroblastoma (11)
- Adult B Acute Lymphoblastic leukemia (1)
- Adult B Acute Lymphoblastic Leukemia (6)
- Adult T Acute Lymphoblastic Leukemia (6)
- Adult T Lymphoblastic Lymphoma (2)
- Adult T-Cell Leukemia/Lymphoma (1)
- Alveolar Rhabdomyosarcoma (4)
- Alveolar Ridge Squamous Cell Carcinoma (1)
- Amelanotic Melanoma (3)
- Ampulla of Vater Adenocarcinoma (1)
- Ampulla of Vater Adenosquamous Carcinoma (3)
- Anaplastic Astrocytoma (3)
- Anaplastic Large Cell Lymphoma (7)
- Askin Tumor (1)
- Astrocytoma (5)
- B Acute Lymphoblastic Leukemia (2)
- B-Cell Non-Hodgkin Lymphoma (5)
- Bare Lymphocyte Syndrome Type 2 (1)
- Barrett Adenocarcinoma (2)
- Benign Prostatic Hyperplasia (1)
- Bladder Carcinoma (14)
- Bladder Squamous Cell Carcinoma (1)
- Bovine Leukemia (2)
- Breast Adenocarcinoma (4)
- Breast Carcinoma (9)
- Breast Ductal Carcinoma (2)
- Burkitt Lymphoma (17)
- Canavan Disease (1)
- Canine Histiocytic Sarcoma (1)
- Cecum Adenocarcinoma (3)
- Central Nervous System Lymphoma (2)
- Cervical Adenocarcinoma (2)
- Cervical Adenosquamous Carcinoma (2)
- Cervical Small Cell Carcinoma (1)
- Cervical Squamous Cell Carcinoma (2)
- Chicken Bursal Lymphoma (2)
- Childhood B Acute Lymphoblastic Leukemia (13)
- Childhood T Acute Lymphoblastic Leukemia (16)
- Childhood T Lymphoblastic Lymphoma (1)
- Cholangiocarcinoma (2)
- Chronic Eosinophilic Leukemia (1)
- Chronic Lymphocytic Leukemia (2)
- Chronic Myeloid Leukemia (23)
- Clear Cell Renal Cell Carcinoma (2)
- Colon Adenocarcinoma (55)
- Colon Carcinoma (34)
- Colorectal Adenocarcinoma (1)
- Colorectal Carcinoma (1)
- Congenital Pure Red Cell Aplasia (1)
- Cutaneous Melanoma (10)
- Dedifferentiated Chondrosarcoma (1)
- Desmoplastic Melanoma (1)
- Diffuse Large B-Cell Lymphoma (28)
- Down Syndrome (2)
- EBV-Related Burkitt Lymphoma (12)
- Embryonal Carcinoma (3)
- Embryonal Rhabdomyosarcoma (3)
- Endometrial Adenocarcinoma (13)
- Endometrial Adenosquamous Carcinoma (2)
- Endometrial Carcinoma (2)
- Endometrioid Stromal Sarcoma (1)
- Epithelioid Hemangioendothelioma (1)
- Epithelioid Sarcoma (3)
- Esophageal Adenocarcinoma (6)
- Esophageal Squamous Cell Carcinoma (41)
- Essential Thrombocythemia (1)
- Ewing Sarcoma (2)
- Extraskeletal Myxoid Chondrosarcoma (1)
- Fanconi Anemia (1)
- Fibrosarcoma (1)
- Follicular Lymphoma (2)
- Gallbladder Carcinoma (2)
- Gallbladder Undifferentiated Carcinoma (2)
- Gastric Adenocarcinoma (6)
- Gastric Adenosquamous Carcinoma (1)
- Gastric Carcinoma (5)
- Gastric Choriocarcinoma (1)
- Gastric Fundus Carcinoma (1)
- Gastric Signet Ring Cell Adenocarcinoma (1)
- Gastric Small Cell Carcinoma (2)
- Gastric Tubular Adenocarcinoma (5)
- Gastroesophageal Junction Adenocarcinoma (1)
- Gestational Choriocarcinoma (1)
- Gingival Squamous Cell Carcinoma (2)
- Glioblastoma (18)
- Gliosarcoma (1)
- Goldfish Erythrophoroma (4)
- Hairy Cell Leukemia (1)
- Hamster Kidney Tumor (1)
- Hamster Pancreatic Ductal Adenocarcinoma (1)
- Hamster Uterine Leiomyosarcoma (1)
- Hepatoblastoma (2)
- Hepatocellular Carcinoma (6)
- Hepatosplenic T-Cell Lymphoma (2)
- Hereditary Thyroid Gland Medullary Carcinoma (1)
- High Grade B-Cell Lymphoma (1)
- High Grade Ovarian Serous Adenocarcinoma (8)
- Hodgkin Lymphoma (9)
- Hypopharyngeal Squamous Cell Carcinoma (2)
- Infectious Mononucleosis (1)
- Intrahepatic Cholangiocarcinoma (6)
- Invasive Breast Carcinoma of No Special Type (12)
- Invasive Breast Lobular Carcinoma (1)
- Kidney Neoplasm (1)
- Kidney Rhabdoid Tumor (1)
- Krukenberg Tumor (1)
- Liposarcoma (1)
- Lung Adenocarcinoma (17)
- Lung Giant Cell Carcinoma (8)
- Lung Large Cell Carcinoma (9)
- Lung Mucoepidermoid Carcinoma (1)
- Lung Non-Small Cell Carcinoma (2)
- Lung Small Cell Carcinoma (25)
- Lung Squamous Cell Carcinoma (9)
- Lymphoblastic Lymphoma (1)
- Malignant Peripheral Nerve Sheath Tumor (1)
- Mantle Cell Lymphoma (5)
- Mature Gastric Teratoma (1)
- Maxillary Sinus Squamous Cell Carcinoma (1)
- Medaka Hepatoma (2)
- Medulloblastoma (3)
- Melanoma (24)
- Meningioma (2)
- Minimally Invasive Lung Adenocarcinoma (1)
- Monophasic Synovial Sarcoma (1)
- Mouse Bladder Transitional Cell Carcinoma (1)
- Mouse Chondrosarcoma (1)
- Mouse Colon Adenocarcinoma (3)
- Mouse Ependymoma (2)
- Mouse Erythroid Leukemia (13)
- Mouse Fibrosarcoma (5)
- Mouse Glioblastoma (1)
- Mouse Hemangioendothelioma (1)
- Mouse Hepatocellular Carcinoma (1)
- Mouse Insulinoma (3)
- Mouse Intestinal Tract Neuroendocrine Adenoma (1)
- Mouse Islet Cell Adenoma (1)
- Mouse Kidney Carcinoma (1)
- Mouse Leukemia (10)
- Mouse Leydig Cell Tumor (1)
- Mouse Lymphoma (8)
- Mouse Mammary Gland Malignant Neoplasm (23)
- Mouse Melanoma (9)
- Mouse Multiple Myeloma (5)
- Mouse Myeloid Leukemia (3)
- Mouse Neoplasm (1)
- Mouse Neuroblastoma (21)
- Mouse Oral Cavity Squamous Cell Carcinoma (1)
- Mouse Osteosarcoma (3)
- Mouse Pituitary Gland Neoplasm (1)
- Mouse Plasmacytoma (1)
- Mouse Precursor T Cell Lymphoblastic Lymphoma/Leukemia (2)
- Mouse Pulmonary Adenoma (1)
- Mouse Pulmonary Malignant Tumor (3)
- Mouse Pulmonary Squamous Cell Carcinoma (1)
- Mouse Rectum Carcinoma (2)
- Mouse Reticulum Cell Sarcoma (2)
- Mouse Sarcoma (1)
- Mouse Teratocarcinoma (8)
- Mouse Thymic Lymphoma (3)
- Mycosis Fungoides (1)
- Myelodysplastic Syndrome (1)
- Myxofibrosarcoma (1)
- Natural Killer Cell Lymphoblastic Leukemia/Lymphoma (2)
- Neuroblastoma (26)
- Oral Cavity Squamous Cell Carcinoma (15)
- Osteoid Osteoma (1)
- Osteosarcoma (15)
- Ovarian Carcinoma (1)
- Ovarian Clear Cell Adenocarcinoma (1)
- Ovarian Endometrioid Adenocarcinoma (4)
- Ovarian Granulosa Cell Tumor (1)
- Ovarian Mucinous Adenocarcinoma (2)
- Ovarian Serous Adenocarcinoma (2)
- Ovarian Serous Cystadenocarcinoma (2)
- Ovarian Small Cell Carcinoma (1)
- Pancreatic Adenocarcinoma (13)
- Pancreatic Carcinoma (5)
- Pancreatic Ductal Adenocarcinoma (12)
- Papillomavirus-Independent Cervical Squamous Cell Carcinoma (1)
- Papillomavirus-Related Cervical Adenocarcinoma (7)
- Papillomavirus-Related Cervical Squamous Cell Carcinoma (4)
- Papillomavirus-Related Endocervical Adenocarcinoma (16)
- Paroxysmal Nocturnal Hemoglobinuria (3)
- Pharyngeal Squamous Cell Carcinoma (1)
- Plasma Cell Myeloma (15)
- Pleural Epithelioid Mesothelioma (5)
- Pleural Sarcomatoid Mesothelioma (2)
- Poorly Differentiated Thyroid Gland Carcinoma (1)
- Primary Cutaneous T-Cell Non-Hodgkin Lymphoma (1)
- Primary Effusion Lymphoma (7)
- Primitive Neuroectodermal Tumor (1)
- Prostate carcinoma (1)
- Prostate Carcinoma (9)
- Rat C-Cell Carcinoma (1)
- Rat Cholangiocarcinoma (1)
- Rat Colon Adenocarcinoma (5)
- Rat Digestive System Neoplasm (1)
- Rat Fibrosarcoma (1)
- Rat Hepatocellular Carcinoma (20)
- Rat Histiocytic Sarcoma (1)
- Rat Insulinoma (2)
- Rat Leukemia (1)
- Rat Leydig Cell Adenoma (1)
- Rat Lung Carcinoma (1)
- Rat Malignant Glioma (4)
- Rat Malignant Meningioma (1)
- Rat Malignant Oligodendroglioma (2)
- Rat Malignant Thymoma (3)
- Rat Mammary Gland Adenocarcinoma (10)
- Rat Neuroblastoma (3)
- Rat Osteosarcoma (2)
- Rat Pituitary Gland Neoplasm (6)
- Rat Prostate Adenocarcinoma (3)
- Rat Rhabdomyosarcoma (1)
- Rat Sarcoma (2)
- Rat Squamous Cell Carcinoma (1)
- Rat Urinary Bladder Transitional Cell Carcinoma (2)
- Rat Urinary System Neoplasm (6)
- Rectal Adenocarcinoma (13)
- Rectosigmoid Adenocarcinoma (1)
- Recurrent Bladder Carcinoma (1)
- Renal Cell Carcinoma (7)
- Renal Pelvis Urothelial Carcinoma (1)
- Retinoblastoma (11)
- Sacral Chordoma (1)
- Sacrococcygeal Teratoma (1)
- Salivary Gland Squamous Cell Carcinoma (1)
- Sezary Syndrome (1)
- Shwachman-Diamond Syndrome (1)
- Skin Squamous Cell Carcinoma (2)
- Splenic Marginal Zone Lymphoma (1)
- Testicular Embryonal Carcinoma (8)
- Testicular Teratoma (2)
- Testicular Yolk Sac Tumor (1)
- Thyroid Gland Anaplastic Carcinoma (10)
- Thyroid Gland Follicular Carcinoma (4)
- Thyroid Gland Papillary Carcinoma (3)
- Thyroid Gland Sarcoma (1)
- Thyroid Gland Squamous Cell Carcinoma (2)
- Tongue Adenosquamous Carcinoma (1)
- Tongue Squamous Cell Carcinoma (6)
- Type I Endometrial Adenocarcinoma (1)
- Ureter Urothelial Carcinoma (1)
- Uterine Carcinosarcoma (2)
- Uterine Corpus Leiomyosarcoma (1)
- Uterine Corpus Sarcoma (2)
- Uveal Melanoma (2)
- Vaginal Melanoma (2)
- Vulvar Melanoma (1)
- Vulvar Squamous Cell Carcinoma (1)
Description: Species: human, Caucasian male 22 years; Tissue: testis; Tumor: carcinoma, embryonal pluripotent; ...
Description: Complex type germ cell tumor of human embryonic testis origin.
Description: Complex type germ cell tumor of human embryonic testis origin.
Description: Complex type germ cell tumor of human embryonic testis origin.
Description: A human embryonal carcinoma cell line derived from a human testicular germ cell tumour.