Spheroid vs. Organoid: Choosing the Right 3D Model for Your Research
For years now, 2D cell culture has been the norm in biomedical research. This flat world on a petri dish is well established, simple, and compatible with a wide variety of research applications. But there's one small problem. The rigid plastic of a petri dish is no real representation of the structure and microenvironment of human tissue in the body. This key disconnect has hindered drug discovery for years, resulting in high attrition rates for many drug candidates that fail in animals and clinical trials.
The good news? The rise of Three-Dimensional (3D) cell culture models is revolutionizing the lab, offering more physiologically relevant systems that bridge the gap between simple 2D monolayers and complex animal models. Spheroids and Organoids are at the top of two powerful 3D technologies with each offering "3D" advantages. With so many overlaps in both capabilities, which technology is right for your research needs? In this overview, we summarize the key differences between Spheroids and Organoids to help you find the right tool for your study.
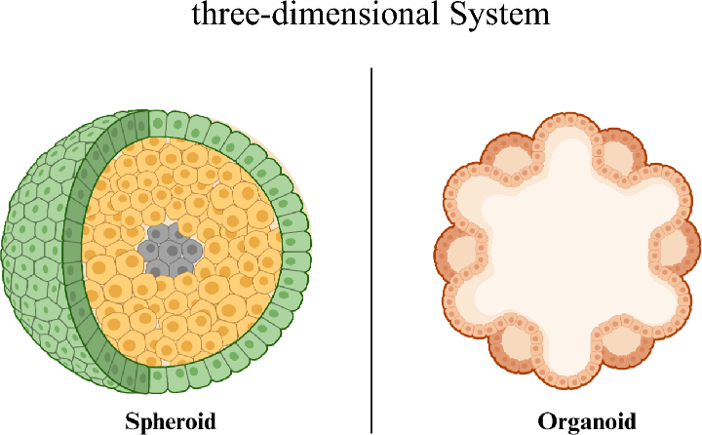
Spheroids or Organoids, what's the difference?
Though both models produce a self-assembled, 3D cellular structure, their cellular source, mode of formation and complexity are inherently different.
What is a Spheroid?
A spheroid is a simple, spherical cellular aggregate.
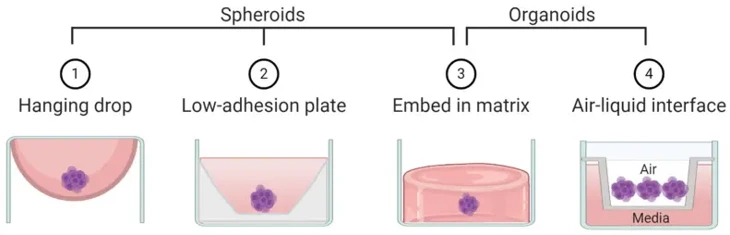
- Formation Mechanism: Spheroids are the product of cell-cell adhesion. They often form in scaffold-free culture systems (ultra-low attachment plates or hanging drop method) in which cells aggregate spontaneously to one another to form a cellular aggregate through surface tension and to reduce adhesion to the substrate.
- Cellular Source: Spheroids can be generated from immortalized cell lines (such as common cancer cell lines e.g. HeLa, MCF-7) or primary cells.
- Culture System: Spheroid culture is a relatively fast and straightforward process, and a defined ECM or particular growth factors aren't strictly necessary (although both can be added). The final structure often (similar to a tumor in vivo) will have gradients of oxygen, nutrients, and metabolites with an actively proliferating outer shell and a quiescent or necrotic core.
- Core Feature: They are simple, non-polarized cellular aggregates.
What is an Organoid?
An organoid is a complex, self-organizing 3D structure which accurately recapitulates the architecture and functionality of the parent organ.
- Formation Mechanism: Organoids are formed through the special property of stem cells or tissue-specific progenitor cells to self-organize and differentiate into multiple cell lineages in vitro. This process is dependent on a carefully balanced medium formulation, including key growth factors and often a supportive ECM scaffold (Matrigel or a defined hydrogel).
- Cellular Source: Derived from pluripotent stem cells (PSCs), adult stem cells (ASCs) or patient derived tumor tissue (often termed 'tumoroids').
- Culture System: Organoid establishment is more complex and time-intensive (often weeks) and requires much more controlled tuning of the culture microenvironment to properly induce differentiation and morphological development, resulting in a defined architecture with lumens, polarity, and a number of specialized cell types.
- Core Feature: They have tissue specific-architecture and organ-like function, with multi-cellular complexity and spatial organization (polarity).
The Core Difference of Spheroid and Organoid
| Category | Spheroids | Organoids |
|---|---|---|
| Cell Source | Immortalized cell lines, primary cells | iPSCs/ESCs, adult stem cells, patient-derived tissue |
| Structural Complexity | Low to moderate | High, with organ-like architecture |
| Cellular Diversity | Typically homogeneous; limited types | Multiple specialized cell types |
| Microenvironment | Gradient-rich; tumor-like | Tissue-like structure with functional domains |
| Culture Requirements | Low-cost, minimal supplements | ECM scaffold + complex medium |
| Throughput | High-throughput compatible | Moderate to low throughput |
| Biological Relevance | Good for tumors or simple tissues | Excellent for modeling physiology and disease |
| Variability | Highly reproducible | Donor-dependent; batch variability common |
| Typical Applications | Drug screening, oncology studies, immune co-culture, toxicology | Disease modeling, development biology, gene editing studies, personalized medicine |
| Scalability | Easy to scale and automate | More difficult to scale; slower growth |
| Formation Time | Hours to days | Days to weeks |
How to Choose the Right 3D Model
1. What is your research objective?
High-throughput drug screening?
Spheroids are ideal due to consistency and scalability.
Mechanistic disease studies or modeling organ development?
Organoids provide tissue-level insight.
Immuno-oncology or tumor microenvironment reconstruction?
Both can be used, but spheroids excel for quick assays, while tumor-derived organoids offer patient specificity.
2. How important is biological fidelity?
Need to recapitulate organ-specific function or structure?
Choose organoids.
Need a simple but physiologically relevant 3D model?
Choose spheroids.
3. What level of reproducibility do you require?
High reproducibility / minimal variability:
Spheroids
Willing to accept donor variability for higher realism:
Organoids
4. What resources and timeline do you have?
Limited budget or rapid turnaround:
Spheroids
Access to stem cell facilities and ECM matrices; longer timelines:
Organoids
5. Do you need patient-specific or personalized data?
General mechanistic studies:
Spheroids
Precision medicine / patient-derived models:
Organoids, especially PDOs.
Conclusion: Choosing the Right Model to Accelerate Your Research
Both spheroids and organoids represent powerful advances beyond traditional 2D culture, offering more realistic microenvironments and improved translational relevance.
- Spheroids deliver speed, scalability, and reproducibility-ideal for drug discovery and high-throughput applications.
- Organoids deliver unmatched biological realism-ideal for disease modeling, developmental biology, and personalized medicine.
The key is aligning your choice with your scientific goals, resources, and the level of complexity your experiment demands.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
|---|---|
| Organoid Models | Creative Bioarray has introduced a variety of disease organoid models to advance research in drug discovery, disease modeling, and regenerative medicine. |
| Organoid Drug Screening | Creative Bioarray provides cutting-edge organoid drug screening solutions that combine innovation, precision, and efficiency. With our expertise, you can accelerate the drug discovery process while obtaining reliable and actionable results. |
| 3D Tumor Spheroid Assay | Creative Bioarray is committed to providing high-quality services and products to support cancer research and drug discovery. Our 3D Tumor Spheroid Assay is a powerful tool for evaluating the efficacy of potential anti-cancer drugs. |
| Custom 3D Cell Culture Services | Creative Bioarray offers 3D cell culture solutions to provide physiologically relevant models. |
References
- Jadhav, S., Rath, S.N. et al. A Review on Multicellular Spheroids and Organoids for Breast Cancer Diagnosis and Therapy. Biomedical Materials & Devices 2025; 3, 771-793.
- Fitzgerald AA, Li E, et al. 3D Culture Systems for Exploring Cancer Immunology. Cancers (Basel). 2020; 13(1):56.