General in vitro Neurotoxicity Test
The central nervous system (CNS) is often considered the most frequent target organ of systemic toxicity. The developing nervous system is particularly vulnerable to chemical insults. Because of the imperceptibility of neurotoxicity, most of the project closures due to neurotoxic issues occur in the clinical phase, while safety issues related to other organs can be addressed much earlier in the drug development process. To control the risk of chemical effects on human health, we used to use in vivo toxicity testing. These traditional paradigms for hazard identification and risk assessment are based on toxicity tests using animal models. Although traditional animal-based paradigms are impractical for screening, the extensive resources and time needed to complete single chemical evaluation do not make these studies amenable to hazard identification in the face of thousands of untested chemicals.
Luckily, in vitro models could be useful for rapid toxicological screening of large numbers of chemicals for their potential to produce toxicity. Such screening could facilitate prioritization of resources needed for in vivo toxicity testing towards those chemicals most likely to result in adverse health effects. Ultimately, new testing paradigms based on in vitro assays have the potential to eliminate the need for traditional animal tests. Cell cultures derived from nervous system tissue have proven to be powerful tools for elucidating cellular and molecular mechanisms of nervous system development and function, and have been used to understand the mechanism of action of neurotoxic chemicals.
With extensive experience and state-of-the-art technologies, Creative Bioarray is offering General in vitro neurotoxicity services to study the negative effects of chemicals on the nervous system. The approach we provide will facilitate the risk evaluation process in drug discovery and to a reduction of animal use.
Advantages
- Various cell types (both cell lines and primary neuronal cultures; human cells or other species) are available to test your chemicals.
- High content and High-throughput screening are available.
- 2D and 3D cultures.
- Multiple endpoints that cover most of the aspects of toxicity evaluation.
Assays available
- Neurite outgrowth: a hallmark of neuronal differentiation and critical determinants of neuronal connectivity since axonal and dendritic processes (collectively called neurites) are a defining characteristic of neuronal morphology.
- Neuron Cytotoxicity: includes apoptosis assay, cell viability assay, and mitochondrial toxicity assay.
- Calcium signaling: a key indicator in the neuronal activity and function. Spontaneous Ca2+ signals are an intrinsic property of differentiating neurosphere-derived precursors. Their frequency may specify neuronal morphology and acquisition of neurotransmitter phenotype.
- Other assays are available on request.
Study Example
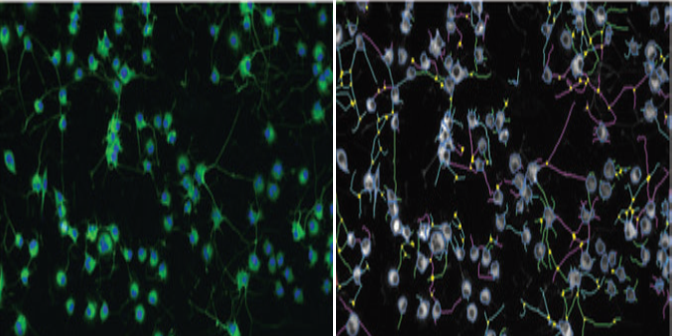 Figure 1. High content images for neurite outgrowth in Neuroscreen-1 cells.
Figure 1. High content images for neurite outgrowth in Neuroscreen-1 cells.
Quotation and ordering
Our customer service representatives are available 24hr a day!
References
- Radio, N., et al. Assessment of chemical effects on neurite outgrowth in PC12 cells using high content screening. Toxicological sciences. 2008, 105.1: 106-118.
- Van T., et al. Translating neurobehavioural endpoints of developmental neurotoxicity tests into in vitro assays and readouts. Neurotoxicology. 2012, 33.4: 911-924.
- Radio, N., and William R. Developmental neurotoxicity testing in vitro: models for assessing chemical effects on neurite outgrowth. Neurotoxicology. 2008, 29.3: 361-376.
- Ciccolini, F., et al. Local and global spontaneous calcium events regulate neurite outgrowth and onset of GABAergic phenotype during neural precursor differentiation. Journal of Neuroscience. 2003, 23.1: 103-111.
Explore Other Options