3D Cell Culture Guide
Traditional cell culture is in 2-dimensions, on plastic dishes or in flasks. It's almost impossible to recapitulate the real conditions cells survive in vivo. Over the past decades, many researchers devoted to looking for new ways to culture cells in a more physiologically relevant manner. Thereby 3D cell culture is generated, which can restore in vivo tissue conditions and processes, as well as provide in vivo like responses. 3D cell culture is an invaluable tool in developmental, cell, and cancer biology. In this guidebook, we will introduce some basic knowledge about 3D cell culture.
What Is 3D culture?
Unlike 2D culture environments, a 3D cell culture is a unique cell culture environment in which cells can grow or interact with their surroundings in three dimensions. In this artificially created system, these 3D cultures are generally grown in bioreactors or small capsules in which the cells can grow into spheroids or 3D cell colonies.
Cells in 3D environment are good models as ‘near-to-in vivo’ systems and give us useful insights in a variety of ways. They have served as a cost-effective screening platform for drug development and testing providing us with a better and more realistic predictive value for safety and risk assessment. 3D cell culture systems have many applications in differentiation studies such as drug discovery & pharmacological studies, cancer research, gene & protein expression studies, etc. For better understanding, the complex cellular physiological mechanisms are reviewed and presented here.
Why Use It?
As is well-known, tissue microenvironment, which includes extracellular matrix (ECM) and surrounding cells, is not only fundamental to tissue formation and function but also crucial in the control of tissue growth and development. Traditional 2D cell cultures lack proper environmental context and structural architecture, which will result in changes in cell functions. When grown in 2D monolayers, normal epithelial cells often lose differentiation characteristics, become highly plastic and show characteristics displayed by tumor cells, while malignant cells often differ from their solid tumor counterparts.
3D cell cultures span the gap between 2D cell cultures and animal models:
- 3D cell culture provides a reproducible, controlled microenvironment that mimics conditions in vivo.
- 3D models still being amenable to facile genetic manipulation, biochemical analysis, and imaging.
- 3D cell cultures enable the studies of questions difficult to address in organisms by creating designed microenvironments in vitro.
- 3D models have become a valid alternative replacement of animal models.
How to Do 3D Cell Culture?
The first 3D cell culture was in 1970s in the form of floating collagen gels, 3D cell culture systems have greatly evolved and are still undergoing development and refinements with the final goal to recreate entire organs in culture. According to cellular input, cell culture format, and 3D microenvironment characteristics and means of production, several key types of 3D cell culture have been recognized.
| Scaffold-Based | Non-Scaffold Based |
|
|
Scaffold Based 3D Cell Culture
Scaffold based 3D culture systems build the cell environment based on physical structures.
• Polymeric Hard Scaffolds
Polymeric hard scaffolds provide a pre-fabricated scaffold or matrices to create a 3-dimensional cell culture system which is designed to mimic the in vivo ECM. Based on this scaffold, cells attach, migrate, and fill the interstices to form 3D cultures.
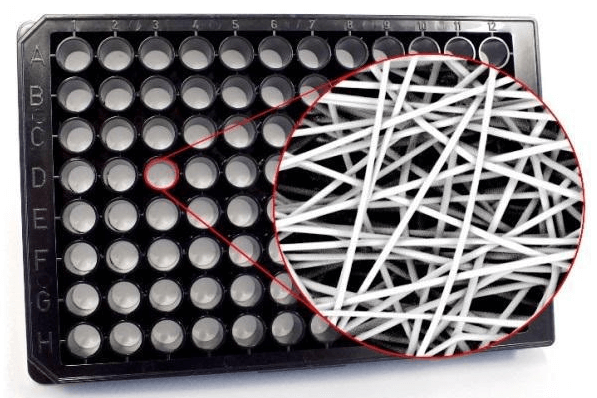 Figure 1. Polymeric 3D scaffold in microplate.
Figure 1. Polymeric 3D scaffold in microplate.
Polymeric hard scaffolds are mainly applied in two research areas incorporated: regenerative medicine and preclinical in vitro testing. In the former, cells grow with the scaffold support and eventually replace degenerative or altered tissue in vivo after transplantation. Currently, scaffolds are used for engineering bone, cartilage, ligament, skin, and vascular. For preclinical in vitro testing, it’s used to model tumors or tissue under a laboratory setting, which provides experiment materials for drug screening assays.
• Biological Scaffolds
In addition to polymeric hard scaffolds, a more natural or biological origin components can also be used as scaffolds. For example, proteins commonly found in the in vivo ECM including but not limited to, fibronectin, collagen, laminin, and gelatin. Enzymatic digestion of decellularized tissues allows these tissue-specific components to be reconstituted into bioactive hydrogels through a physical crosslinking of collagen.
Current methods require cells to be mixed with scaffold proteins in a hydrogel prior to plating in a microplate well, or added to previously formed scaffolds. This method allows cells to restructure the microenvironment and release signaling molecules, and eventually, form a proper homeostatic state.
• Micropatterned Surface Microplates
Each 3D culture microplates contains micrometer-sized compartments that are regularly arrayed on the bottom of each well. These microplates allow for spheroid or cluster growth through selection of adhesion molecules, meanwhile, it also controls the size. This method allows us to observe more accurate in vivo cell activity, which is opening up doors to high throughput drug screening.
Non-Scaffold Based 3D Culture Systems
Scaffold-free 3D culture systems place emphasis on facilitating the self-assembly of 3D cells, rather than using a physical support.
• Hanging Drop Microplates
Hanging drop plates (HDP) take advantage of gravity encouraging 3D growth of the cells in a downwards form after seeded in wells. HDP is different from traditional plates, it has an opening bottom. Cells in media can be dispensed from the top of HDP wells like conventional microplate, while a discrete droplet of media sufficient for cellular aggregation hangs over the opening bottom by surface tension. Cells in the suspended media droplet aggregate over the course of hours to days creating the spheroid structure.
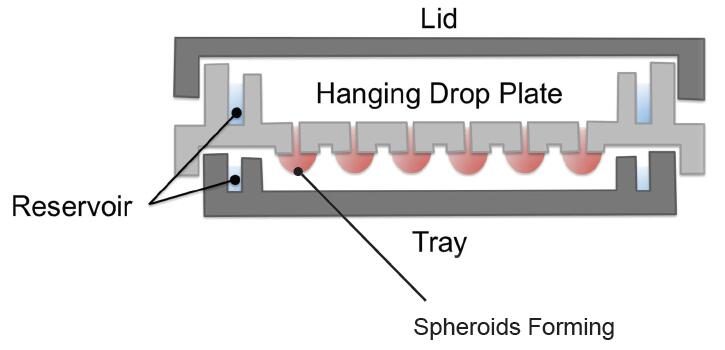 Figure 2. Diagram of hanging drop microplates.
Figure 2. Diagram of hanging drop microplates.
If you want to harvest a long-term culturing of spheroids and to conduct assays, you just need to transfer the spheroids to a second plate capable of containing larger media or buffer volumes which have higher nutrient levels, for propagation periods reaching days or even weeks. It's at present one of the most cost-effective methods for 3D cell culture, since it only requires the cells and media, and is compatible with automated liquid handling devices.
• Spheroid Microplates containing Ultra-Low Attachment (ULA) coating
Different from HDP's opening bottom, these spheroid microplates have the typical well shape and depth. The only difference is that the bottom is coated with a ULA surface to minimize cell adherence to allow spheroid formation. Three types of well bottoms are available: round, tapered and v-shaped geometry. All these special well bottoms ensure the creation of consistent, single spheroids and help to position the spheroids in the middle of the well.
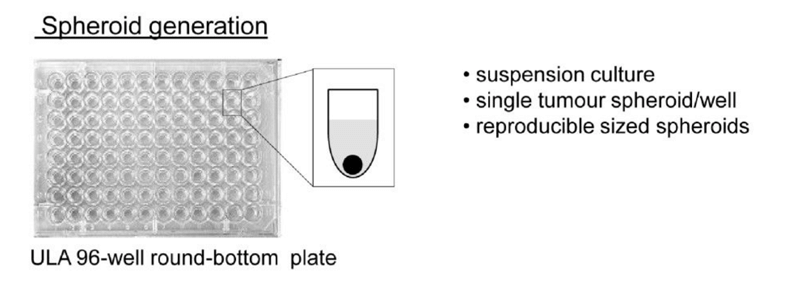 Figure 3. Ultra-low attachment (ULA) 96-well round-bottomed plates were used to generate suspension cultures of reproducibly sized, single spheroids in each well. (Vinci M, 2012)
Figure 3. Ultra-low attachment (ULA) 96-well round-bottomed plates were used to generate suspension cultures of reproducibly sized, single spheroids in each well. (Vinci M, 2012)
Since spheroid microplates provide the similar volume capacity to general plates, spheroid aggregation, propagation, and experimental procedures can be carried out in the same plate; without the need of transferring to a second microplate.
• Microfluidic 3D Cell Culture
Microfluidic platforms add a perfusive flow in the 3D cell culture system. Firstly, cells are maintained in a compartment disparate physicals barriers (such as glass or silicon, polymers, and polystyrene (PS)) or non-physical barriers (including collagen, matrigel, or gelatin). Then, a perfusive flow is introduced into the cellular environment to allow continuous nutrition and oxygen introduction as well as waste removal through culture medium.
Microfluidic systems are also used for varied 3D cell culture applications including stem, primary, and cancer cells.
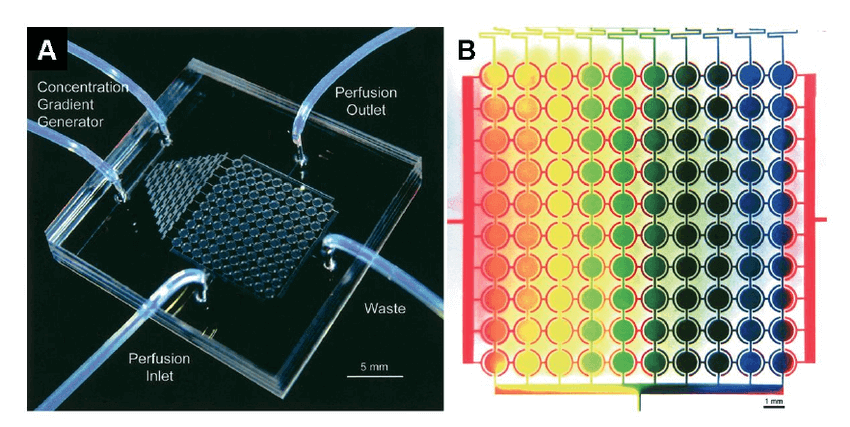 Figure 4. A perfusion microfluidic cell culture array for high-throughput cell-based assays. (A) Photograph of the microfluidic cell culture array with concentration gradient generator and 10 × 10 microchambers on a 2 cm × 2 cm device. (B) Concentration gradient across 10 columns generated from the concentration gradient generator at the top of the device. All chambers were initially filled with a red dye. Blue and yellow dyes were then loaded from two separate ports at the top of the gradient generator. This array can be used to test drugs at various dosages. (Zang R, 2012)
Figure 4. A perfusion microfluidic cell culture array for high-throughput cell-based assays. (A) Photograph of the microfluidic cell culture array with concentration gradient generator and 10 × 10 microchambers on a 2 cm × 2 cm device. (B) Concentration gradient across 10 columns generated from the concentration gradient generator at the top of the device. All chambers were initially filled with a red dye. Blue and yellow dyes were then loaded from two separate ports at the top of the gradient generator. This array can be used to test drugs at various dosages. (Zang R, 2012)
The Significance and Future Directions
Three-dimensional cultures systems are a very important advancement in cell culture techniques. If the conventional 2D culture systems are used to answer the question about complex cellular physiology and how cells function and respond to stimuli, the 3D culture approach has taken us a step closer to the in vivo conditions and help us investigate the real reactions in vivo. Augmented with advancements in cell imaging and analytical systems, the applications of new scaffolds and matrices, cells are increasingly being grown as three-dimensional models.
Getting Started with Creative Bioarray
Cells are important materials for various studies. Technological advancements are important to address emerging complex challenges and the way cells are cultured in vitro is an area of intense activity. Studies in cell biology over the last decade have shown that cells aggregated in three dimensions can provide more physiologically relevant cellular responses compared to 2D cells culture.
We provide 3D cell culture based research services in three different areas.
- In our 3D cell culture service, custom 3D cell cultures are prepared for all researchers as a strong tool to proceed their projects. In addition, for some researchers who have the ability to conduct 3D culture by themselves, we offer comprehensive 3D culture products.
- For 3D based drug discovery, we offer a set of services including but not limited to: high content image service, ATP content assay, 3D hepatotoxicity service, and 3D cardiovascular toxicity service.
- Based on our extensive knowledge and perfect platforms, we have an application on oncology while 3D oncology service is a part of it. In this department, greater accuracy and increased throughput drug tests, such as drug efficacy test, 3D angiogenesis assay, 3D invasion assay and co-culture models, are offered based on 3D cell culture.
Creative Bioarray is dedicated to becoming a leading company in the area of cell biology, providing the most appropriate methods for different types of studies. The expert team at Creative Bioarray provides high-quality consulting and solutions around the clock in order to solve any problems you encounter. We hope Creative Bioarray could be your first choice in any situation. We look forward to cooperating with you soon.
References
- Elliott N T, Yuan F A N. A review of three‐dimensional in vitro tissue models for drug discovery and transport studies. Journal of pharmaceutical sciences. 2011, 100(1): 59-74.
- Vinci M,; et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC biology. 2012, 10(1): 29.
- Zang R,; et al. Cell-based assays in high-throughput screening for drug discovery. International Journal of Biotechnology for Wellness Industries. 2012, 1(1): 31.
- Haycock, John W. 3D cell culture. Humana Press. 2011.