Adherent and Suspension Cell Culture
Suspension Cells
Suspension Cells: Suspension cells can float and reproduce in culture medium during in vitro cultivation without requiring attachment to solid surfaces. The cells exhibit spherical shapes or round forms and maintain consistent dimensions while showing limited interaction with surrounding cells. The cells appear suspended in the culture medium while remaining unattached to the culture flask bottom when viewed under a microscope. Generally, suspension cells mainly originate from cells in the blood and lymphatic tissues, such as lymphocytes and white blood cells, as well as certain tumor cells and hybridoma cells constructed from myeloma. Because their cultivation does not require a substrate these cells can proliferate quickly and pass conveniently through cell cultures which makes them ideal for growing in large quantities.
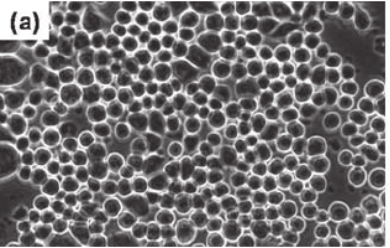 Fig. 1. Morphology of suspension mammalian cells (Eibl R, Eibl D, et al., 2009).
Fig. 1. Morphology of suspension mammalian cells (Eibl R, Eibl D, et al., 2009).
Adherent Cells
Adherent cells need to attach to solid surfaces like glass or plastic for growth and division. As adherent cells grow, they attach to the bottom of the flask where they create a densely packed layer that typically appears flat or polygonal because of adhesion molecules linking neighboring cells. The main sources for these cells are human body tissues and organs including fibroblasts, epithelial cells, muscle cells, liver cells and kidney cells. When the density of adherent cells reaches a specific level contact inhibition slows their growth rate until it completely stops. To maintain growth proper functioning, adherent cells need consistent passaging.
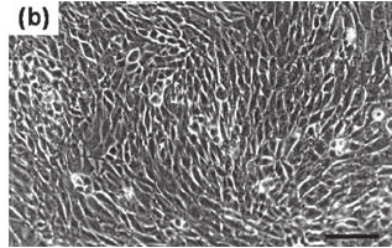 Fig. 2. Morphology of adherent mammalian cells (Eibl R, Eibl D, et al., 2009).
Fig. 2. Morphology of adherent mammalian cells (Eibl R, Eibl D, et al., 2009).
| Comparison Dimension | Suspension Cell Culture | Adherent Cell Culture |
| Cell Growth | Freely floating in liquid | Requires attachment to a solid surface |
| Applicable Cells | Non-adhesion-dependent cells | Adhesion-dependent cells |
| Culture Vessels | Regular culture bottles/flasks/bioreactors | TC-treated dishes/bottles/microcarrier systems |
| Operation | Simple | More complex |
| Passage Method | Direct dilution/concentration | Enzymatic separation followed by seeding |
| Lag Phase | Short | Possibly longer |
| Cell Density | Limited by medium concentration | Limited by surface area |
| Application Areas | Biopharmaceuticals, vaccines, etc. | Tissue engineering, gene therapy, etc. |
| Advantages | Operationally simple, easy expansion | Mimics in vivo environment, suitable for interaction studies |
| Limitations | Minimal cell interactions | Scale-up constraints, complex operations |
Suspension Cell Culture
Methods:
1. Prepare the medium: Choose serum-free or serum-containing media suitable for suspension cells (e.g., RPMI 1640, DMEM/F12). Add antibiotics (e.g., penicillin/streptomycin) and necessary growth factors.
2. Initial Cultivation: Directly inoculate frozen or passaged suspension cells into culture bottles/flasks without pre-treatment. The inoculation density is usually 1×10⁵~5×10⁵ cells/mL.
3. Passage Steps:
- Direct Passage Method: When cell density reaches 80%~90%, directly distribute the original culture medium into new bottles, adding fresh medium, and decide on replenishment based on density.
- Centrifugal Passage Method: If cell condition is poor, centrifuge (900 rpm, 3 minutes) to collect cell pellets, discard the supernatant, resuspend in fresh medium, and distribute to new bottles.
Key points:
- Observe cell morphology (e.g., aggregation, fragments) and medium color changes daily.
- Passaging is needed when cell density is too high to prevent nutrient depletion or waste accumulation.
- Suspension cells are susceptible to contamination, requiring strict aseptic techniques.
- Maintain uniform cell suspension and gas exchange via shaking, magnetic stirring, or bottle turning (>2400 r/h), avoiding excessive mechanical damage.
- Avoid directly pouring medium; transfer gently using a pipette.
- Handle aggregated cell clumps gently to disperse, preventing local hypoxia.
Adherent Cell Culture
Methods:
1. Prepare medium and surface: Choose a medium suitable for adherent cells (e.g., DMEM, MEM). Use TC-treated dishes/bottles or add microcarriers (e.g., Cytodex).
2. Inoculation: Inoculate frozen or passaged adherent cells onto pre-serum-coated culture surfaces, allowing static cultivation. Adjust inoculation density based on cell type, ensuring sufficient cell attachment.
3. Passage Steps:
- Digestion Treatment: Remove old medium, wash with PBS, add trypsin-EDTA (0.25%), digest at 37℃ until cells round up and detach, terminating with serum-containing medium.
- Centrifugation and Resuspension: Centrifuge (900 rpm, 3 minutes) to collect cells, resuspend, and seed into new culture bottles at a desired ratio.
Key points:
- Culture containers need tissue culture treatment (e.g., positive charge modification) to promote cell adherence.
- If attachment is weak, assess medium, trypsin digestion time, and seeding density.
- Insufficient attachment area may lead to cell detachment or slow growth.
- Growth is inhibited once a monolayer is formed; timely passaging is needed (usually when density reaches 80%~90%).
- Scale-up is limited to surface area; increase the number of culture bottles or use microcarriers (e.g., patented microcarrier suspension culture) for scaling.
- Strict control over digestion time is required to avoid excessive cell damage.
- Add serum (e.g., fetal bovine serum) to the medium for attachment factors and nutrients.
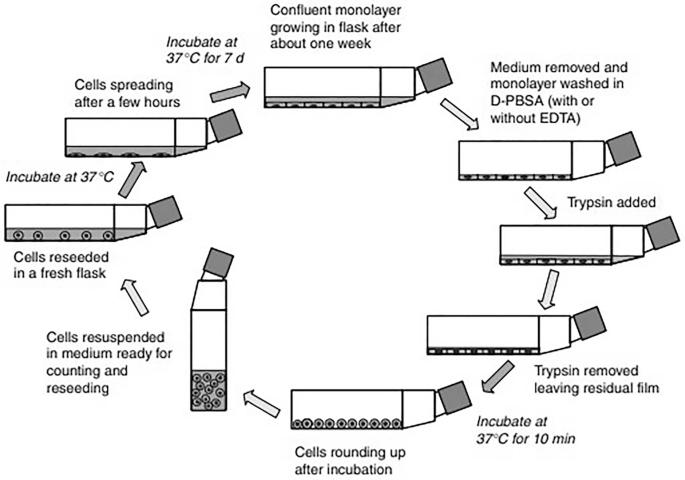 Fig. 3. Subculturing in monolayer cultures (Pancham P, Jindal D, et al., 2023).
Fig. 3. Subculturing in monolayer cultures (Pancham P, Jindal D, et al., 2023).
FAQ
How to handle adherence in suspension cells?
Minor adherence is normal: Suspension cells (like THP-1) may slightly adhere due to gravity when stationary. Gently swirling or tapping the culture flask can resuspend them without special treatment.
Investigate high adherence rate: If the proportion of adherent cells exceeds 20%, check the following:
- Incubator Settings: Ensure stable temperature, CO₂ concentration, and humidity.
- Medium Composition: Verify that serum and nutrients are adequate and fresh.
- Culture Vessels: Ensure that no adhesion-promoting coatings (like polylysine) are used.
Change culture vessels: If vessel surface properties are suspected to cause adherence, transfer cells to untreated regular culture flasks.
How to handle cell debris in suspension cell culture?
- Low-speed centrifugation to remove debris: Centrifuge at 800-1000 rpm for 3-5 minutes, discard the supernatant, and retain the cell pellet. Note: Use cautiously at low cell densities to avoid excessive cell loss.
- Optimize operations: Reduce the number and intensity of pipetting to avoid mechanical damage. Use a cell strainer to filter the suspension and remove larger debris.
- Check culture conditions: Confirm that the medium is not degraded (e.g., abnormal pH, nutrient depletion). Avoid frequent passaging or overly high cell density.
What to do when suspension cells aggregate?
Cell aggregation may lead to local hypoxia and uneven nutrient distribution. Address this by:
- Increasing the shaking or stirring speed of the culture system to ensure even cell distribution in the medium.
- Using appropriate chemical dispersing agents or adjusting the medium composition to reduce cell adhesion.
- Regularly and gently dispersing aggregated cells.
How to address difficulties in cell attachment?
- Check if the medium and surface treatment are suitable for adherent cell growth; use TC-treated culture containers.
- Ensure sufficient serum and attachment factors in the medium.
- Adjust the seeding density appropriately to ensure sufficient cell contact with the surface.
- Increase static time to allow more time for cells to attach.
What are the optimization methods for slow cell growth?
- Optimize medium formulation to ensure all essential nutrients and growth factors are provided.
- Maintain appropriate temperature, humidity, and CO₂ concentration for the culture environment.
- Regularly check and maintain sterile conditions in the culture setup.
- Adjust the seeding density to an optimal level to avoid high or low densities affecting cell growth.
- Consider adding specific growth-promoting additives or growth factors to enhance cell proliferation.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| Primary Cell Media | Creative Bioarray offers high-quality cell culture media that meet various requirements. |
References
- Eibl R, Eibl D, et al. Mammalian cell culture technology: an emerging field. Springer Berlin Heidelberg. 2009.
- Pancham P, Jindal D, et al. Inoculation and Passaging of Adherent and Suspension Cells. In: Animal Cell Culture: Principles and Practice. Techniques in Life Science and Biomedicine for the Non-Expert. Springer, Cham. 2023.