Strategies for Enrichment of Circulating Tumor Cells (CTCs)
Despite the very low concentration of CTCs observed in circulation, a variety of technologies have been disclosed for the identification and isolation of CTCs from blood samples of cancer patients. Due to the short half-life of the CTCs in bloodstream (estimated from 1 to 2.4 h), CTCs isolation devices must be efficient enough to preserve potential CTCs for further investigation.
Two main directions were chosen to help isolate CTCs:
- Because the CTCs are shed from the primary solid tumor, biological properties such as expression of cell surface adhesion molecule (ex: EpCAM) or deficiency in expression of blood-type surface antigen markers (ex: leukocyte marker CD45, granulocyte marker CD15) could be used to distinguish CTCs from blood cells.
- Physical properties, such as size, density, or electric charge, could be used as selection criteria for CTC isolation. The approaches in CTC-isolating devices include size-based filtration exclusion, density-based centrifugation isolation, biological affinity- based immuno-magnetic beads selection, antibody- functionalized microfluidic platforms, surface-modified cell enrichment chips, acoustic-based cell selection devices, electric-charge-based electrophoresis, and a combination of multiple properties for CTC isolation.
Although there are different properties and advantages amongst the diverse platforms for capturing and isolating CTCs, size-based exclusion, density- based isolation, and antibody-based affinity capture remain the three major methods used for the isolation and identification of CTCs.
Size-Based Exclusion for CTCs Isolation
One of the first approaches to isolate CTCs from blood samples was based on the difference in size between tumor cells and blood cells.
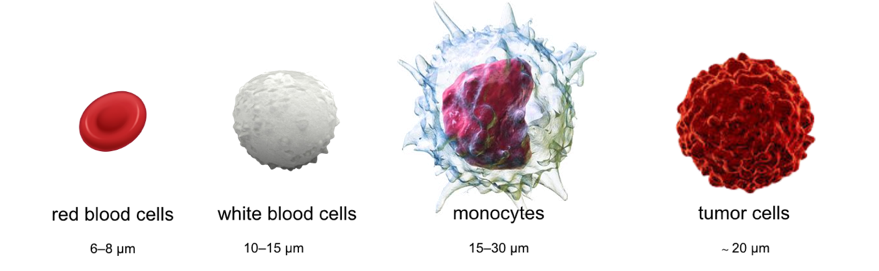 Figure 1. The average size of red blood cells (RBCs), white blood cells (WBCs), and monocytes is 6–8 μm, 10–15 μm, and 15–30 μm, respectively. Tumor cells have a high nuclear to cytoplasm ratio and higher stiffness, with an average size of 20 μm. Their larger size, compared to WBCs, led to the use of marker-free, size-based filtration for CTC isolation directly from blood.
Figure 1. The average size of red blood cells (RBCs), white blood cells (WBCs), and monocytes is 6–8 μm, 10–15 μm, and 15–30 μm, respectively. Tumor cells have a high nuclear to cytoplasm ratio and higher stiffness, with an average size of 20 μm. Their larger size, compared to WBCs, led to the use of marker-free, size-based filtration for CTC isolation directly from blood.
The size-based exclusion technology is featured in rapid separation and non-selected marker-free isolation for the CTCs, though the technology may not be able to distinguish monocytes from CTCs in blood samples. The early implementation of size-based exclusion used a commercially available, porous filter membrane to isolate the CTCs from blood samples. Problems that may need to be addressed to meet the critical utility for the size-based isolation and characterization approach include non-even pore sizes, low pore density, and the inability to isolate and hold the CTC.
Recently, size-based CTC isolation using a microfabricated microfilter made by etching a polymer membrane with reactive ion etching to give precisely controlled pore sizes and density was demonstrated. The isolation by size of epithelial tumor cells (ISET) technology is one of the earliest marker-free technologies to isolate CTCs based on the larger size of epithelial tumor cells by directly filtering blood through a calibrated, polycarbonate Track-Etch-type membrane with 8-μm-diameter pores. The ISET technology can detect CTCs in 80% of samples from stage III-IV in non-small cell lung cancer (NSCLC), and provides label-free CTC samples for further morphological, immunocytological, and genetic characterization of individual CTCs.
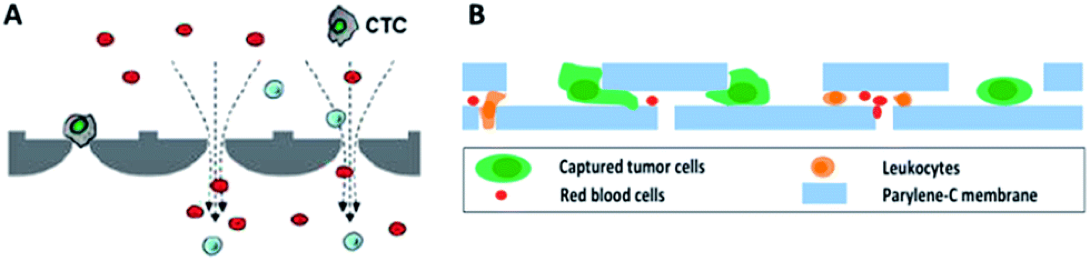 Figure 2. Working principle of microfiltration-based devices for CTC isolation. (A) Size-selective microcavity array. (B) Separable bilayer microfiltration device. (Song, Y. 2017)
Figure 2. Working principle of microfiltration-based devices for CTC isolation. (A) Size-selective microcavity array. (B) Separable bilayer microfiltration device. (Song, Y. 2017)
Density-Based Centrifugation for Isolation of CTCs
Blood contains various types of cells, including abundant RBCs, nucleated WBCs (including eosinophils, basophils, neutrophils, lymphocytes, and monocytes), and heterogeneous CTCs populations of varying number density in cancer patients.
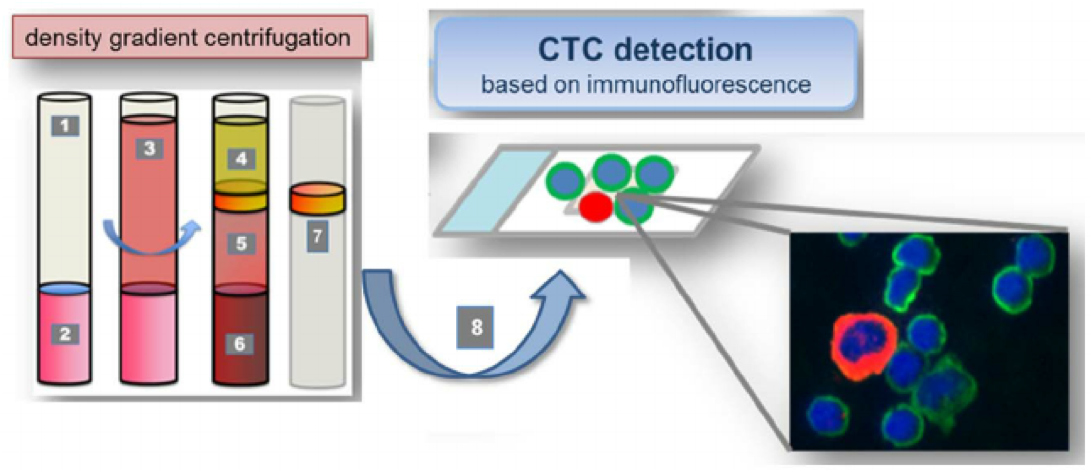 Figure 3. CTCs were isolated from peripheral venous blood using density gradient centrifugation. 1. Leucosep tube: 2. Separation media; 3. Whole blood/PBMNC mixture; 4. Plasma; 5. Separation media after centrifugation; 6. Erythocytes; 7. Buffy coat incl. CTCs; Cell suspension containing CTCs for Cellspin and multi-immunofluorescence staining. Cell suspensions were carefully spun onto glass slides. The presence of CTC was verified by immunofluorescence staining against pan-CK (epithelial), N-cadherin (mesenchymal), CD133 (stemcell-like); counterstaining with CD45 (hematopoietic) and DAPI (nucleus). (Weller, P. 2014)
Figure 3. CTCs were isolated from peripheral venous blood using density gradient centrifugation. 1. Leucosep tube: 2. Separation media; 3. Whole blood/PBMNC mixture; 4. Plasma; 5. Separation media after centrifugation; 6. Erythocytes; 7. Buffy coat incl. CTCs; Cell suspension containing CTCs for Cellspin and multi-immunofluorescence staining. Cell suspensions were carefully spun onto glass slides. The presence of CTC was verified by immunofluorescence staining against pan-CK (epithelial), N-cadherin (mesenchymal), CD133 (stemcell-like); counterstaining with CD45 (hematopoietic) and DAPI (nucleus). (Weller, P. 2014)
Density-based centrifugation technologies take advantage of the difference in cell density between blood cells to isolate nucleated cells directly from whole blood. By using mixtures of high molecular weight sucrose polymers and sodium diatrizoate, such as Ficoll-Paque, CTCs can be isolated from whole blood. Density-based separation provides rapid and non-selective methods, without additional processing, that enable obtaining viable cells directly from blood samples. But the density-based centrifugation methods show very low purity of the isolated CTCs due to the concomitant isolation of nucleated WBCs with the CTCs. Recently, Williams et al. have successfully established a patient-derived xenograft mouse model by using CTCs isolated from prostate cancer patients through density- based centrifugation accompanied with RBC lysis and additional CD45-depletion. The patient-derived mouse xenograft model provides a model platform for further investigation of CTCs in functional studies or molecular characterization in vivo.
Biological Affinity-Based Selection for Capture of CTCs
Most solid tumors derive from epithelial origin cells, which express EpCAM on the cell surface to facilitate cell-cell contact and adhesion. Identification of epithelial - origin tumor cells in the blood stream by using the anti-EpCAM antibody as an affinity-based method to capture CTCs provides good sensitivity and specificity in the isolation of CTCs from blood. However, selective capture of EpCAM-positive cells in the blood stream may result in a bias in the CTC population selection and may possibly lead to the loss of other CTC populations, such as those that have gone through EMT.
It has been reported that most CTCs express both epithelial and mesenchymal markers on the cell surface, suggesting that EpCAM-based affinity capture could isolate the majority of the population of both epithelial and mesenchymal types of CTCs disseminated from solid tumors. Since the release of the CellSearch® platform, affinity-based devices for more sensitive and specific capture of CTCs have been developed.
 Figure 4. The CellSearch® platform uses anti-EpCAM to isolate CTCs and has been used as a standard for CTC studies in various types of cancers, including prostate, breast, ovarian, colorectal, lung, pancreatic, and head and neck cancer.
Figure 4. The CellSearch® platform uses anti-EpCAM to isolate CTCs and has been used as a standard for CTC studies in various types of cancers, including prostate, breast, ovarian, colorectal, lung, pancreatic, and head and neck cancer.
The MagSweeper system was developed using 4.5 μm magnetic beads coated with antibody against human EpCAM for the isolation of CTCs directly from blood samples. The system shows higher CTC detection rates (70%) in metastatic breast cancer than the CellSearch® platform and is feasible for downstream molecular analysis such as single cell profiling. In addition, Nagrath et al. developed the CTC-chip for more efficient and selective separation of the CTCs from blood samples. The CTC-chip features as a microfluidic system consisting of anti-EpCAM-conjugated microspot arrays and is operated under predesigned laminar flow conditions without prelabeling of the blood samples. The platform reaches 99% identification rate in metastatic lung, prostate, pancreatic, breast, and colon cancer patients with high CTC capture number and approximately 50% purity.
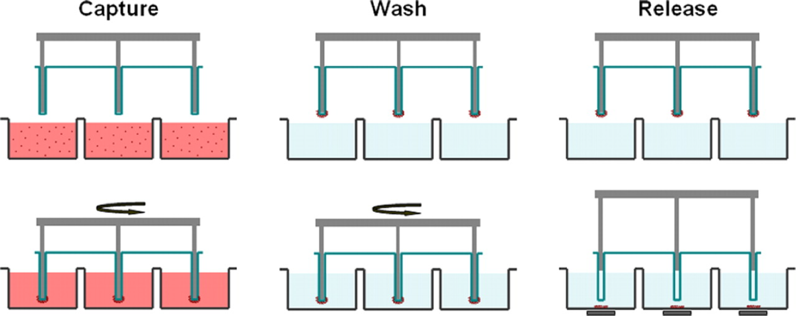 Figure 5. Schematic of the MagSweeper process. The diluted blood samples, which are prelabeled with magnetic particles, are loaded into the capture wells. The magnetic rods covered with plastic sheaths are swept through the well in concentric circular loops at a level 1.5 mm above the bottom of the wells. After sweeping through the whole area of the capture wells, the sheathed magnets are washed in a circular loop to remove loosely bound contaminating cells. The rods are then immersed into a new buffer solution and disengage from the plastic covers. The external magnets located under the wells facilitate release of labeled cells and excess magnetic particles. Another round of capture-wash-release is performed to eliminate the majority of remaining contaminant cells entrapped within excess magnetic particles.
Figure 5. Schematic of the MagSweeper process. The diluted blood samples, which are prelabeled with magnetic particles, are loaded into the capture wells. The magnetic rods covered with plastic sheaths are swept through the well in concentric circular loops at a level 1.5 mm above the bottom of the wells. After sweeping through the whole area of the capture wells, the sheathed magnets are washed in a circular loop to remove loosely bound contaminating cells. The rods are then immersed into a new buffer solution and disengage from the plastic covers. The external magnets located under the wells facilitate release of labeled cells and excess magnetic particles. Another round of capture-wash-release is performed to eliminate the majority of remaining contaminant cells entrapped within excess magnetic particles.
Although these devices demonstrated high efficiency in capturing CTCs directly from blood, releasing intact and viable cells from the devices is still a challenge. In addition to the techniques describe above, Magbanua et al. have also reported a method that combines immunomagnetic enrichment and fluorescence-activated cell sorting (IE/FACS) for the specific isolation of CTCs using two independent EpCAM specific antibodies.
Combination of Multiple Properties for Purification of CTCs
The use of a combination of technologies for CTC separation provides an opportunity to capture and isolate CTCs with higher sensitivity, specificity, and efficiency. The CTC-iChip isolates CTCs based on a combination of several features including size exclusion, affinity-based negative selection, and magnetic separation to obtain a population of pure CTCs directly from whole blood samples.
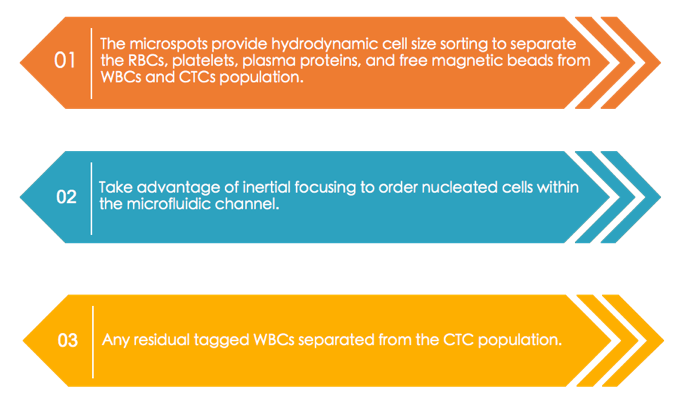
This negative selection is accomplished by magnetic deflection of pre-mixed CD45 and CD15 immunomagnetic beads, with any residual WBCs attached to them. The CTC-iChip has demonstrated up to 95% cell recovery of various cell lines and identified 90% of cancer recurrence in prostate cancer patients based on triple stained CTC enumeration. The device shows efficient isolation with high purity of captured cells and enables further molecular characterization such as single cell RNA expression validation.
Similarly, Kirby et al. developed a geometrically enhanced differential immunocapture (GEDI) device for CTC isolation that features a combination of both size-based exclusion and affinity-based capture for the PSMA-positive CTCs from castration resistant prostate cancer blood samples. In comparison with the CellSearch® system, the GEDI device shows a 2-400-fold increase in the number of CTCs captured in the same patient, indicating a remark- able improvement in the efficiency of CTC capture.
Summary
Although each CTC capturing technology has drawbacks in either CTC population bias or impurity in isolation of the cells, the count of isolated CTCs shows significant correlation with clinical outcome and prognosis prediction, as well as cancer progression and therapeutic treatment efficacy. The determination of more specific markers or isolation strategies for CTC isolation/purification will benefit the clinical utility of future application in blood-based cancer diagnosis.
Related Service
References
- Meng S.; et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004, 10(24):8152–8162.
- Mitchell MJ, King MR. Fluid shear stress sensitizes cancer cells to receptor-mediated apoptosis via trimeric death receptors. New J Phys. 2013, 15:015008.
- Miyamoto DT.; et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015, 349(6254):1351–1356.
- Williams ES.; et al. Generation of prostate cancer patient derived xenograft models from circulating tumor cells. J Vis Exp. 2015, 105:53182.
- Armstrong AJ.; et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res.2911, 9(8):997–1007.
- Song, Y.; et al. Enrichment and single-cell analysis of circulating tumor cells. Chemical Science. 2017, 8(3), 1736-1751.
- Weller, P.; et al. Detection of circulating tumor cell subpopulations in patients with head and neck squamous cell carcinoma (HNSCC). PloS one. 2014, 9(12), e113706.
- Talasaz, A. H.; et al. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proceedings of the National Academy of Sciences. 2009, 106(10), 3970-3975.