Seizure Models
The development of new antiepileptic drugs (AED) relies heavily on the preclinical use of animal models to establish efficacy and safety before the first human trials. This method has made essential contributions to the development of many clinically effective AEDs. During the development of the new AED, animal models of epileptic seizures have multiple applications. In genetic models, genetic lesions (or spontaneous mutations) can cause spontaneous seizures. In the induction model, chemical, electrical, or acoustic stimulation can cause acute or chronic seizures, depending on the purpose of the experiment and the method of induction.
Creative Bioarray provides multiple in vivo models of epilepsy or seizures to help our customers develop new drugs on either chronic or acute seizures.
Models of Seizures or Epilepsy
Genetic Models
Creative Bioarray uses CRISPR-Cas9 technology to provide customized gene editing animal models related to epilepsy diseases. Genetic models have significant advantages in studying recurrent spontaneous epilepsy, and they are more conducive to studying the molecular mechanisms of disease occurrence and development.
Induced Models
- Pentylenetetrazole-Induced Seizure (PTZ)
Pentylenetetrazol (PTZ) is a GABA receptor antagonist used to create standard chemically induced epilepsy models. Among all animal models of epilepsy and epilepsy, pentylenetetrazol-induced epilepsy is classified as a generalized epilepsy model (compared to partial or focal epilepsy). It produces myoclonic seizures, which mimic absence seizures. As a generalized epilepsy model, it has characteristics different from the MES epilepsy model.
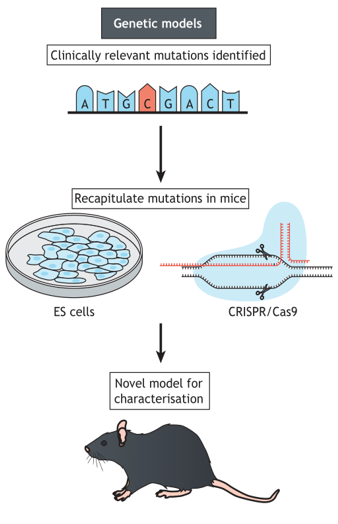 Figure 1. Genetic models.
Figure 1. Genetic models.
(Marshall et al., 2021)
- Maximal Electroshock (MES)
The MES model is classified as a generalized tonic-clonic epilepsy model among all animal models of epilepsy. When screening anticonvulsant drug candidates, it is an excellent tool for evaluating antiepileptic properties compared to focal or partial epilepsy models (such as the 6 Hz model).
- 6-Hz Psychomotor Seizure
 Figure 2. Maximal Electroshock.
Figure 2. Maximal Electroshock.
(Castel-Branco et al., 2009)
The 6 Hz seizure model is classified as partial or focal seizures (psychomotor seizures). This psychomotor epilepsy program is a model used to test the efficacy of compounds to inhibit certain forms of seizures. It is an excellent screening supplement for generalized epilepsy models such as PTZ or MES when characterizing antiepileptic drug candidates.
- Lithium Pilocarpine Status Epilepticus
The lithium-pilocarpine-induced rat model has often been used in status epilepticus (SE) studies. Pilocarpine is used to initiate seizures. Animals were pretreated with lithium (LiCl) to enhance the reliability of seizure induction.
- Kainic acid (KA)
KA is an agonist of ionotropic KA receptors, and it is a cyclic analog of L-glutamate. Tt induces prolonged excitatory responses in cortical neurons following microiontophoretic application in rats. KA could induce robust depolarizations and eventually cell death, central to temporal lobe epilepsy (TLE). The animal model of TLE induced by KA is characterized by a latent period followed by refractory spontaneous seizures, as in human TLE.
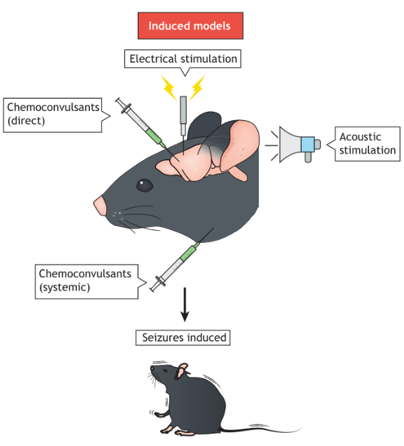 Figure 3. Induced models.
Figure 3. Induced models.
(Marshall et al., 2021)
- Kindling
Epilepsy is a chronic condition with recurrent spontaneous seizures. Kindling is a chronic model of epilepsy where repetitive and intermittent administration of sub-convulsant chemical/electrical stimuli can lead to progressive amplification of seizures, culminating in generalized seizure activity. Chemical kindling involves the administration of certain chemical convulsant substances systemically (e.g., PTZ). Electrical kindling builds epilepsy models by repeatedly stimulating specific brain areas (such as the amygdala) within a certain threshold.
Species
- Rat
- Mouse
Available Assays
- Cytotoxicity assays
- Measurement of cytokines
- Flow cytometric analysis of cell death
- Measurement of serum liver enzymes
- Hematoxylin and eosin staining (H&E)
- Immunohistochemistry
- Quantitative real-time PCR
- TUNEL stain
- Western blot analysis
- Gene expression analysis
- Immunofluorescence
Applications
- Discovery of new AEDs
- Characterization of anticonvulsant activity spectrum of new AEDs
- Specific models for pharmacoresistant seizures
- Evaluation of whether the efficacy of new AEDs changes during chronic treatment
- Comparison of side effects of new AEDs
- Estimation of the effective plasma concentration of the new AED used in the first clinical trial
- Discovery of antiepileptogenic or disease-modifying treatments
Quotation and ordering
If you have any special needs or questions regarding our services, please feel free to contact us. We look forward to cooperating with you in the future.
References
- Castel-Branco, M. M.; et al. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Methods and Findings in Experimental and Clinical Pharmacology, (2009), 31(2), 101–106.
- Marshall, G. F.; et al. Modelling epilepsy in the mouse: Challenges and solutions. Disease Models & Mechanisms, 14(3). (2021).
Explore Other Options