In Vitro Mammalian Cell Micronucleus Test (OECD 487)
- Service Details
- Features
- FAQ
- Explore Other Options
Genotoxicity is a core indicator in the safety assessment of drugs, chemicals, cosmetics, and industrial materials, directly related to potential carcinogenicity and mutagenic risks. Micronuclei are small nuclei separated from the main nuclei of cells, produced during telophase of mitosis or meiosis by lagging chromosome fragments or whole chromosomes, and not incorporated into a daughter nucleus. Thus, the presence of micronuclei indicates genotoxicity and chromosomal instability.
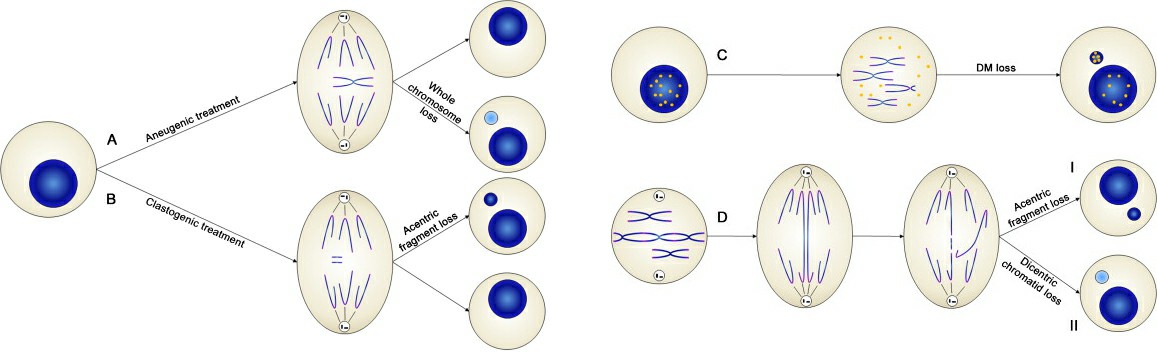 Fig. 1. Mechanisms of micronuclei formation (Terradas M, Martín M, et al., 2010).
Fig. 1. Mechanisms of micronuclei formation (Terradas M, Martín M, et al., 2010).
The in vitro mammalian cell micronucleus test (OECD 487), a globally recognized standard method, efficiently evaluates the genotoxic risk of test substances by detecting micronuclei formed from DNA damage or chromosomal breakage. With stricter global regulations (such as ICH, FDA, EPA), this test has become essential in drug non-clinical studies (GLP), chemical registration (REACH), and consumer product safety evaluations.
Creative Bioarray's In Vitro Mammalian Cell Micronucleus Test Service
Workflow
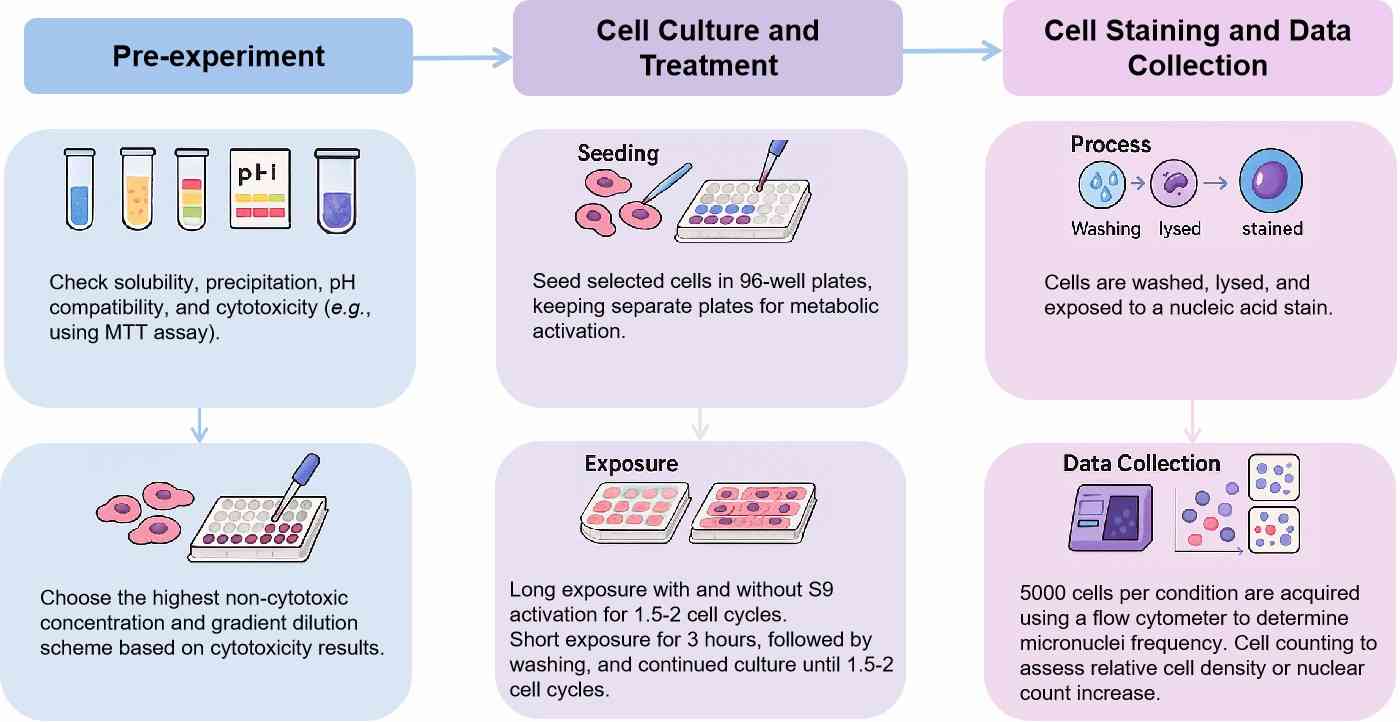
Protocol
| In vitro mammalian cell micronucleus test | Details |
| Cell types |
|
| Concentration | ≥3 effective concentrations + negative control/positive control |
| Exposure Time |
|
| Metabolic system | Aroclor-1254 induced rat liver S9 |
| Compound requirements | 100 μL DMSO* solution, at 200-fold highest concentration, kept at 0.5% DMSO or equivalent solid compound amount |
Custom Services
We also provide customized services, including:
- Multi-concentration gradient testing (e.g., 0.1–1000 μg/mL), dose range optimization.
- Combined genotoxicity endpoint detection (e.g., Ames test, chromosomal aberration test), provide comprehensive toxicity assessment reports.
- Special sample handling (e.g., solubilizer screening for poorly soluble substances, nanoparticle dispersion technology).
Features

Full Compliance Assurance
GLP laboratory system + QA independent review, data traceable to original records, supporting global submissions.

Expert Toxicology Support
Results interpretation by toxicologists with over 15 years of experience, aiding in the optimization of subsequent experimental strategies.
Flexible Custom Solutions
Support for combined testing (e.g., with AMES test, chromosomal aberration test) to reduce overall costs.

Rapid Response Mechanism
Dedicated project manager for full project follow-up, real-time feedback at key stages, ensuring zero delay in project delivery.
FAQ
1. What is micronucleus?
A micronucleus is a small nuclear structure remaining in the cytoplasm of daughter cells after cell division, mainly caused by two types of damage. The first type is caused by chromosome fragments without centromeres, following double-strand DNA breaks, failing to be pulled to cell poles during mitosis, forming micronuclei. The second type results from spindle malfunctions or centromere defects causing entire chromosomes to lag, forming micronuclei containing whole chromosomes. Increased micronucleus frequency directly indicates a substance's ability to damage genomic stability (DNA or chromosomes).
2. What are the applications of the in vitro micronucleus assay?
- Drug Development: Non-clinical genetic toxicity combination testing (ICH S2(R1)) to assess the carcinogenic risk of candidate compounds. Supports FDA/EMA drug market applications, verifying no potential mutagenicity.
- Chemical Safety Assessment: A required test item for chemical registration under REACH regulations to identify the genotoxic hazards of industrial raw materials. Compliance testing for EPA chemical control (TSCA).
- Cosmetics and Consumer Products: Validates ingredient safety through micronucleus testing as required by EU cosmetics regulations (EC 1223/2009). Screens mutagenicity of food contact materials (e.g., plastic additives).
- Environmental Toxicology: Evaluates the genotoxic impact of pollutants (e.g., heavy metals, pesticides) on ecosystems.
- Nanomaterials and Novel Materials: Detects genomic stability risks of nanoparticles and engineered materials, supporting the safety evaluation of nanotechnology products.
3. How to handle abnormal positive control results?
Abnormal positive control results may arise from multiple factors and require systematic investigation. If positive controls show no response, first verify the activity of the metabolic activation system (e.g., S9 mix) with an AMES test to check normal metabolic function. Also, inspect the storage conditions and preparation time of positive control substances (e.g., cyclophosphamide) to prevent reagent degradation leading to inefficacy. If positive control micronucleus frequencies are lower than expected, consider whether exposure time is insufficient or cell cycles are unsynchronized. Extending treatment time to 1.5 times the cell cycle or using synchronization methods (e.g., serum starvation) ensures cells are in the same division stage. For abnormally elevated background micronucleus rates, which may stem from accumulated spontaneous cell damage or experimental operation contamination, replacing cell lines with low spontaneous mutation rates (e.g., TK6) and rigorously partitioning operations to avoid cross-contamination is recommended.
4. What are the special requirements for testing nanomaterials?
The unique physicochemical properties of nanomaterials (e.g., size effects, surface reactivity) pose special challenges for test design:
- Dispersion Control: Nanoparticles tend to agglomerate, requiring ultrasound, surface modification, or biocompatible dispersants (e.g., serum proteins) to ensure uniform distribution.
- Dose Setting: Based on solubility, surface charge, and cytotoxicity data of the material, dose-incremental methods (e.g., 5, 10, 20 μg/mL) and precipitation controls are used.
- Metabolic Activation Considerations: Nanomaterials may act directly on the nucleus through endocytosis, requiring pre-experimental verification of the necessity of the S9 mix.
- Slide Reading Standards Adjustment: Nanoparticles may induce atypical micronuclei (e.g., linear, ring-like), requiring supplementary confirmation with fluorescence in situ hybridization (FISH) or transmission electron microscopy (TEM).
- Negative Control Optimization: In addition to solvent control, it is recommended to add the same type of nanomaterial (e.g., inert SiO₂) as an additional negative control to exclude non-specific interference.
Reference
- Terradas M, Martín M, et al. Genetic activities in micronuclei: is the DNA entrapped in micronuclei lost for the cell? Mutat Res. 2010. 705(1):60-7.
Explore Other Options