Establishment of Drug-resistant Cell Lines
The drug-resistant cell models are developed in the laboratory by repeatedly exposing cancer cells grown in cell culture to drugs. Surviving daughter resistant cells are then compared to the parental sensitive cells by using cell viability/proliferation assays such as MTT, CCK-8, or clonogenic assays. The establishment of drug-resistant cancer cell lines might be one of the useful model systems for studying the molecular mechanisms leading to cancer drug resistance. However, most of the published scientific research papers poorly explained the methods of resistance induction in details. This protocol will provide a useful guide for researchers interested in producing drug-resistant cell lines.
Materials and Equipment
| HEPG2 cell line | Cisplatin (CDDP) |
| Cell counting kit-8 (CCK-8) | 0.25% trypsin-EDTA |
| RPMI 1640 medium | Microplate reader |
| 96-well plates | Incubator |
Assay Procedure
- Determination of inhibitory concentrations
The inhibitory concentration known as IC50, is the optimal concentration at which the survival rate of cells is the lowest after exposure to an appropriate anticancer drug. Determination of this concentration (IC50) is the basis for the selection of any drug resistant cells. Briefly, cells at the logarithmic growth phase (80% confluence) were harvested and seeded at a density of 104 cells/well in 96-well plates and incubated overnight to ensure proper adherence. Subsequently, the drug-free medium was replaced with pre-warmed complete supplemented fresh medium containing 0 µM to 20 µM of CDDP. The cells were incubated for 24 hours at 5% CO2 and 37˚C in a humidified atmosphere. After 24 hours of incubation, cell viability assessment was conducted by using cell counting kit-8 (CCK-8). The cell viability was calculated as a measure of the optical density (OD) of treated cells relative to the untreated controls, excluding the OD of blank controls. A dose-dependent response curve was plotted to establish the inhibitory concentrations (IC50) of CDDP.
- Selection of drug-resistant cells
Clinical method of treatment was adopted in the selection of cells for resistance. The parental HEPG2 cell line was treated in pulse for 4 to 6 hours at the IC50 of CDDP. Induction was repeated 6 times, whilst allowing the cells to reach at least 70% confluence between induction intervals. After 6 complete cycles of induction, selected cells were maintained in drug-free RPMI 1640 medium with appropriate supplements, and passaged at the attainment of 70%-80% confluence. No further experiment was performed on the cells until after 4 weeks maintenance in drug-free medium, whilst the stability of the developed resistance was tested.
- Test for resistance
The stabilities of the selected clones were tested after 2 weeks, 1 month and 3 months of drug withdrawal. Briefly, selected clones were harvested at exponential growth phase using 0.25% trypsin-EDTA, and the cells were seeded in 96-well plate at the concentration of 104 cells/well, in triplicates. Plates were maintained at 37˚C in a humidified atmosphere of 5% CO2. After overnight incubation, cells were incubated for another 24 hours in RPMI 1640 medium containing appropriate concentration of CDDP (0-20 µM). Following the 24 hours incubation, the cells were further incubated in the presence of 10 µL of CCK-8 per well for 2 hours. The optical densities were measured and the new IC50 was obtained from a dose-dependent cytotoxicity curve. The difference in IC50 between the resistant clone and the parental cell line determines the degree of resistance.
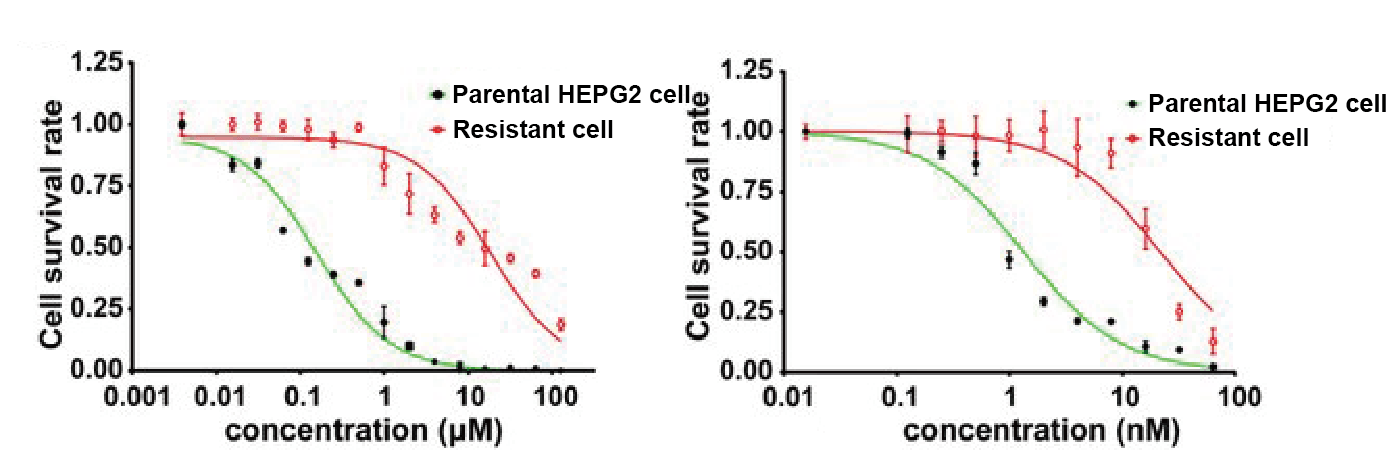 Figure 1. Drug sensitivity assay of HEPG2 cell and resistant cell to CDDP and 5-FU
Figure 1. Drug sensitivity assay of HEPG2 cell and resistant cell to CDDP and 5-FU
References
- Benedict Onyekachi Odii, et al.; Pharmacological isolation of experimental models of drug-resistant hepatocellular carcinoma cell line. Journal of Cancer Therapy, 2012, 3: 216-221.
- McDermott M, et al.; In vitro development of chemotherapy and targeted therapy drug-resistant cancer cell lines: a practical guide with case studies. Front Oncology, 2014, 4: 40.
Cell Services:
Cell Line Testing and Assays: