
Human Villous Trophoblasts (HVT)
Cat.No.: CSC-7693W
Species: Human
Source: Placenta
Cell Type: Trophoblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human villous trophoblasts derived from the villi of the human placenta. The placenta functions as an essential organ for transporting materials between mother and fetus and features primary structures called villi and intervillous spaces. The trophoblast cells surround villi and infiltrate the maternal uterine lining which leads to the formation of placental villi structures. These trophoblasts are divided into two main subtypes: villous cytotrophoblasts (CTB) and extravillous trophoblasts (EVTs). Villous cytotrophoblasts remain within the villi producing the syncytiotrophoblast and hormones while extravillous trophoblasts migrate into maternal tissues to aid placental implantation and blood vessel formation.
Placental villous trophoblasts exhibit rapid cell division during early-stage development. Scientific studies show that these cells continue to divide actively even when oxygen levels are low because this trait supports their essential function during embryo implantation and the establishment of the placenta. Exogenous growth factors such as EGF and IGF activate the MAPK signaling pathway which results in significant proliferation of villous trophoblasts. TB medium together with specific media solutions supports extended in vitro cultivation of villous trophoblast cells. Villous trophoblasts serve as essential models for studying placental development and examining pregnancy-related disease mechanisms. Scientists use villous trophoblasts to examine how placental diseases develop including abnormal implantation of the placenta and pregnancy-induced hypertension syndrome.
Specific PPARγ Agonist and Antagonist Regulate H3K4me3 and H3K9ac in Human Trophoblast Cells
Preeclampsia stands as a common pregnancy disorder which emerges after 20 weeks of gestation through hypertension and organ dysfunction and causes high death rates among mothers and newborns. Because existing therapies fail to cure PE researchers must explore the root causes of the disease. Meister et al. analyzed the expression of peroxisome proliferator-activated receptor γ (PPARγ) and retinoid X receptor α (RxRα), a binding heterodimer playing a pivotal role in the successful trophoblast invasion, in the placental tissue of preeclamptic patients.
Researchers evaluated PPARγ's effects on histone modifications H3K4me3 and H3K9ac in trophoblasts by using Ciglitazone as PPARγ agonist and T0070907 as antagonist. After 24 hours of incubating Human Villous Trophoblasts (HVT) and EVT cells with these compounds, researchers performed histone modification and PPARγ level analysis through double immunofluorescence. HVT cells showed reduced PPARγ levels following Ciglitazone treatment but higher levels when treated with T0070907 as indicated in Figure 1. Ciglitazone treatment led to significant reduction of H3K4me3 fluorescence intensity with p-value of 0.04 while T0070907 treatment caused significant increase (Fig. 2A and B). H3K9ac intensity demonstrated equivalent patterns where it decreased with Ciglitazone (p = 0.005) and increased with T0070907 (p = 0.004; Fig. 2C and D).
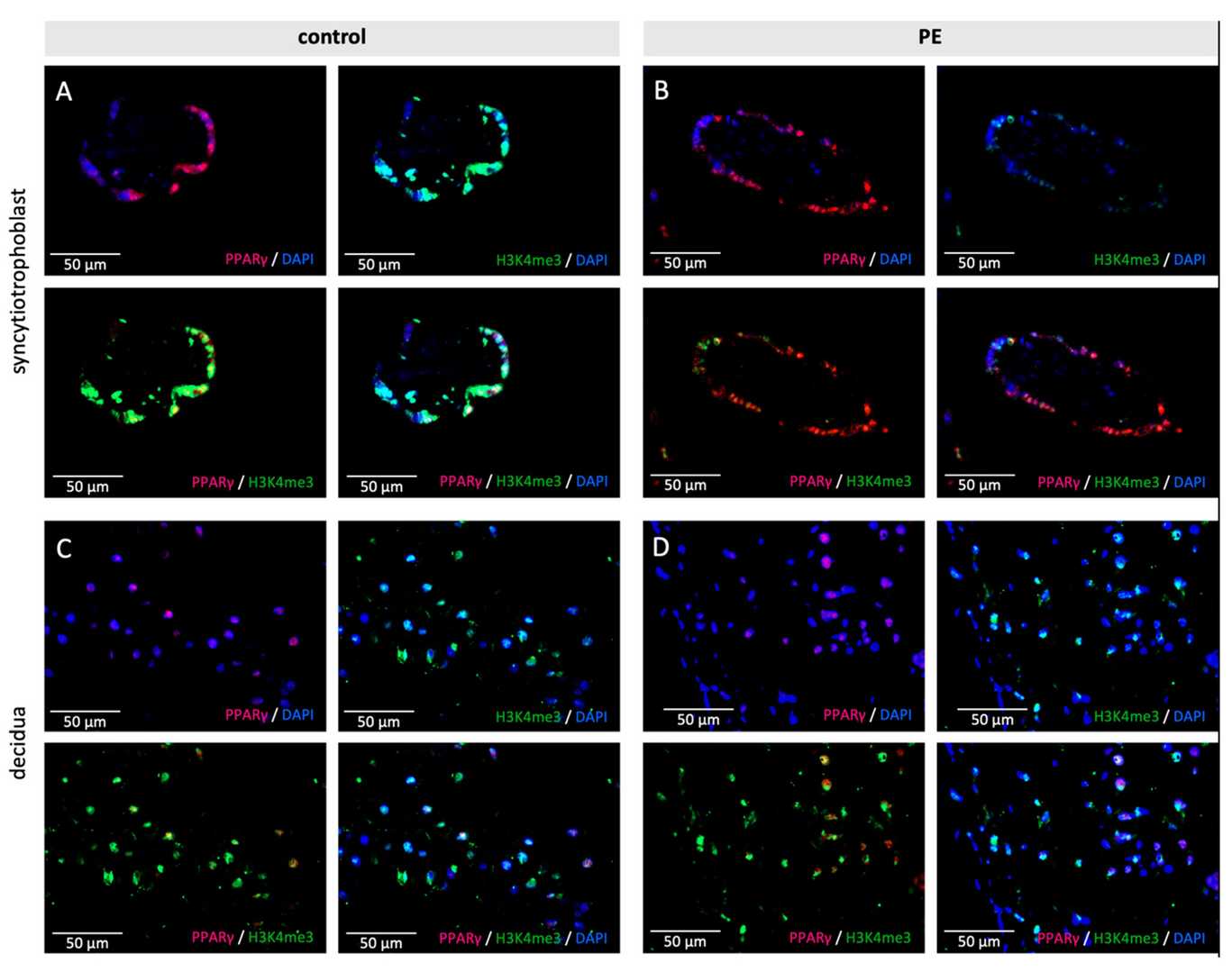 Fig. 1. Examples of staining results of the immunofluorescence of PPARγ and H3K4me3 in control (A,C) and PE placentas (B,D) in the syncytiotrophoblast (A,B) and the decidua (C,D) (Meister S, Hahn L, et al., 2021).
Fig. 1. Examples of staining results of the immunofluorescence of PPARγ and H3K4me3 in control (A,C) and PE placentas (B,D) in the syncytiotrophoblast (A,B) and the decidua (C,D) (Meister S, Hahn L, et al., 2021).
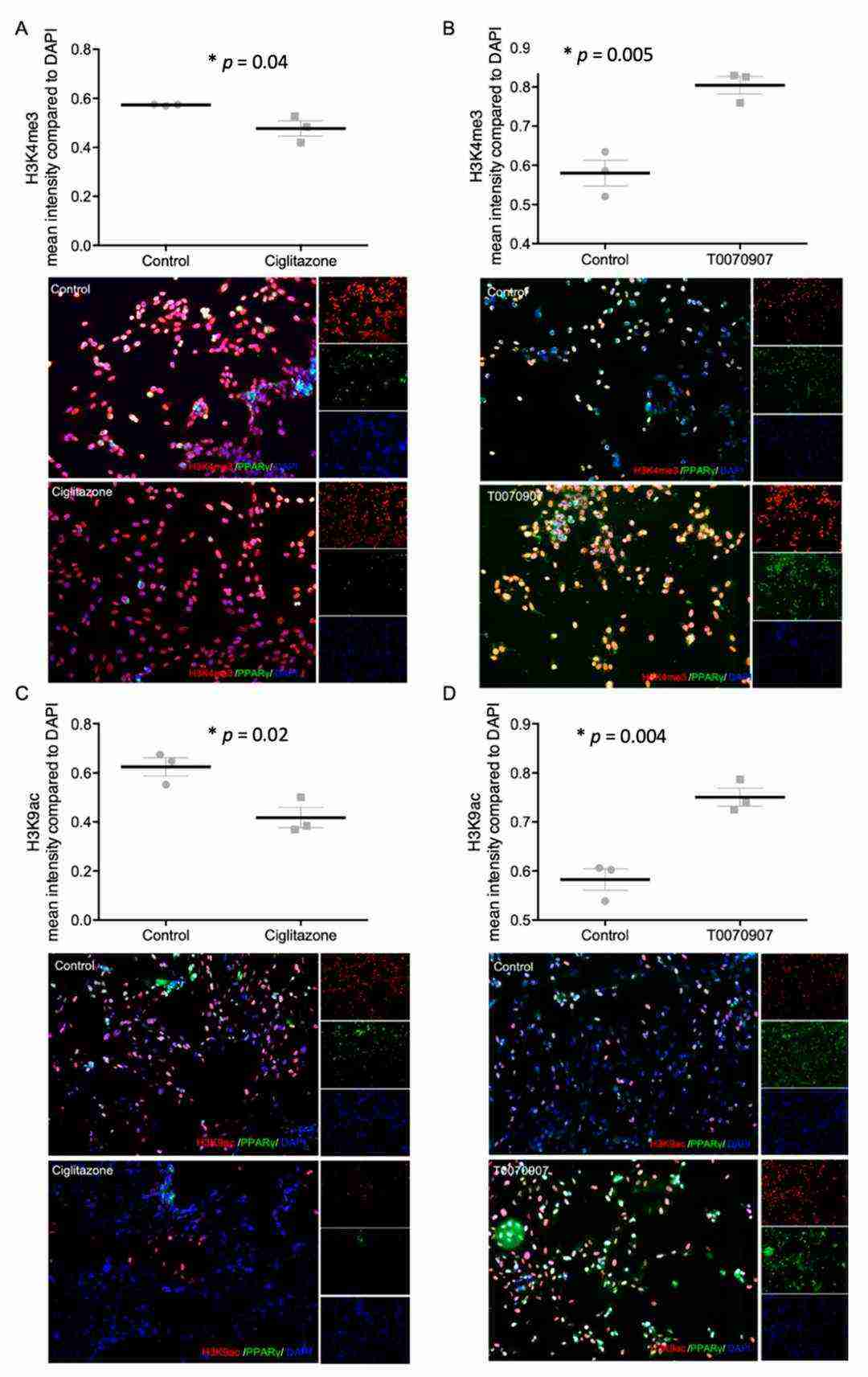 Fig. 2. Staining results of histonemodifications H3K4me3 (A,B) and H3K9ac (C,D) and PPARγ after incubation with Ciglitazone (A,C) (20 mM) and T0070907 (B,D) (Meister S, Hahn L, et al., 2021).
Fig. 2. Staining results of histonemodifications H3K4me3 (A,B) and H3K9ac (C,D) and PPARγ after incubation with Ciglitazone (A,C) (20 mM) and T0070907 (B,D) (Meister S, Hahn L, et al., 2021).
Gal-2 Promotes the Histone Modifications H3K4me3 and H3K9ac in HVT Cells
Preeclampsia represents a significant pregnancy health threat because researchers have yet to understand its pathophysiology or develop effective treatments. Hahn et al. investigated how galectin-2 (Gal-2) affects reduced transcription-promoting histone modifications (H3K4me3, H3K9ac) in PE for understanding its role in trophoblasts and syncytialisation.
They found that Gal-2 promotes the histone modifications H3K4me3 and H3K9ac in BeWo cells. Based on the results of the BeWo cell culture, incubation with Gal-2 was repeated with Human Villous Trophoblasts (HVT) cells (Fig. 3). A significant increase in H3K4me3 was observed compared to untreated controls (p = 0.020, Mann–Whitney U test), with individual group differences (p = 0.044, Kruskal–Wallis test) and a strong correlation with Gal-2 levels (r = 0.735, p < 0.001, Spearman's Rho). However, no significant changes were noted for H3K9ac expression between treated and untreated HVT cells (p = 0.684, Mann–Whitney U test), nor between individual groups (p = 0.900, Kruskal–Wallis test), and no correlation with Gal-2 (p = 0.769, Spearman's Rho). Despite this, a descriptive analysis indicated increased H3K9ac after Gal-2 treatment (1.52 ± 0.822 vs. 1.25 ± 0.540) and slight elevation with varying Gal-2 concentrations.
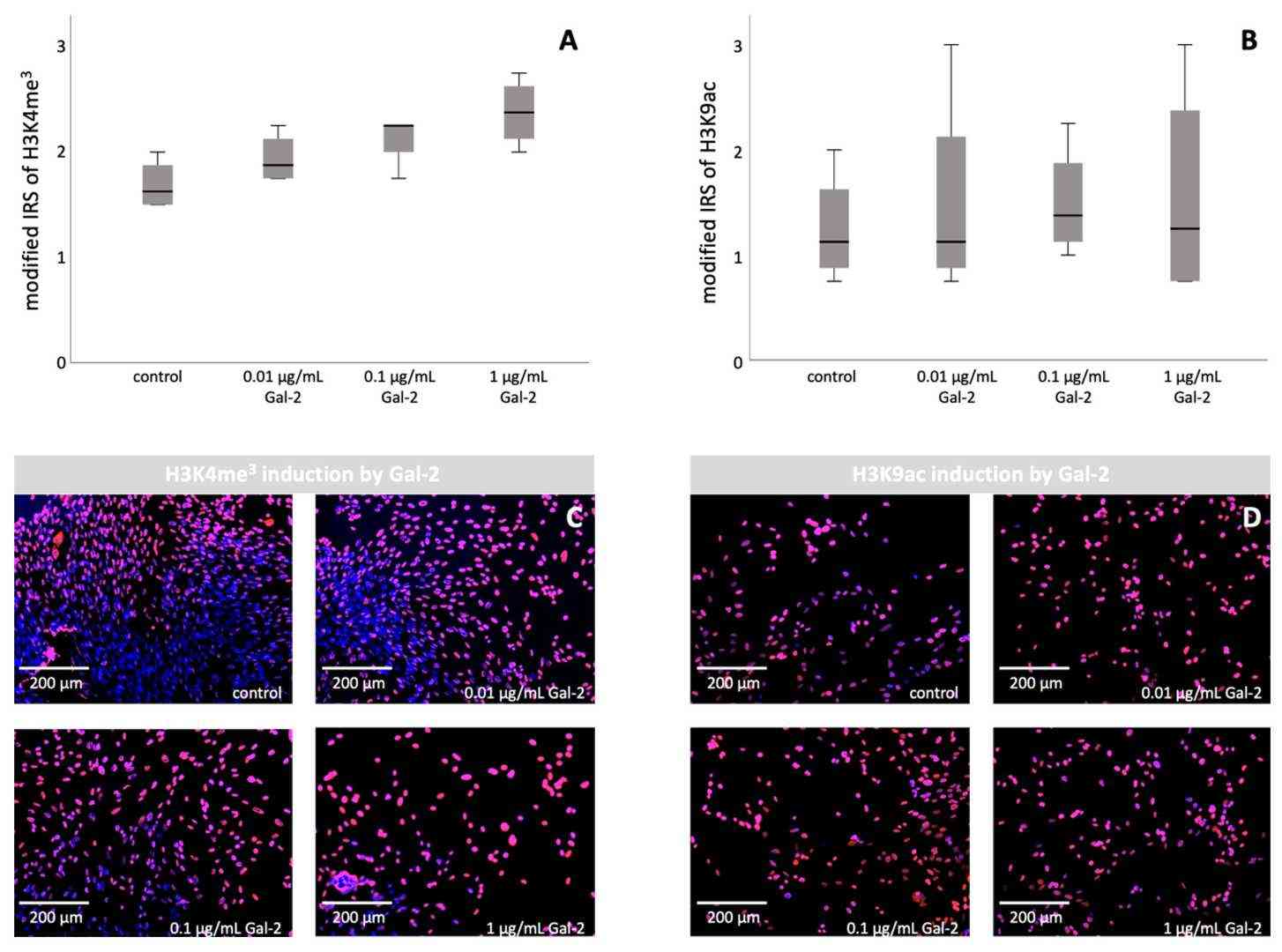 Fig. 3. Induction of H3K4me3 and H3K9ac by increasing the concentration of Gal-2 in HVT cells: staining results of immunocytochemistry of HVT cells (Hahn L, Meister S, et al., 2022).
Fig. 3. Induction of H3K4me3 and H3K9ac by increasing the concentration of Gal-2 in HVT cells: staining results of immunocytochemistry of HVT cells (Hahn L, Meister S, et al., 2022).
According to our experts, the cells are suitable for the study of cell differentiation and organogenesis, thus probably can also be used to study syncytiotrophoblast formation. I'm attaching the datasheet of this product for your reference. Hope it helps.
We are sorry, but the protocols for the isolation is for our internal use thus cannot be revealed to others. Thanks a lot for your understanding.
Ask a Question
Write your own review
