
Human Skeletal Muscle Progenitor Cells (Myoblasts)
Cat.No.: CSC-C3196
Species: Human
Source: Skeletal Muscle
Cell Type: Myoblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Skeletal Muscle Progenitor Cells (myoblasts) are primarily derived from human skeletal muscle tissue. These cells, located within the skeletal muscle tissue, are key to muscle development and regeneration. Myoblasts usually exist in the basal layer of skeletal muscles and become activated by damage or stimuli which leads them to transform into mature muscle fibers. Myoblasts exist primarily in embryonic muscle tissues during fetal development but migrate to skeletal muscle regions after birth. Additionally, specific differentiation protocols enable human induced pluripotent stem cells (hiPSCs) to become myoblasts which serve as an in vitro research model.
Myoblasts primarily function to generate and regenerate skeletal muscle tissue. Myoblasts repair muscle damage by transforming into mature muscle fibers and creating skeletal muscle fibers throughout developmental processes. Furthermore, Myoblasts maintain muscle microenvironment homeostasis through the secretion of growth factors and extracellular matrix components. Myoblasts serve as valuable tools for in vitro and in vivo research into skeletal muscle degenerative diseases like muscular dystrophy and their treatment methods. In tissue engineering myoblasts can be integrated with biomaterials including Matrigel to develop three-dimensional models of muscle tissue that simulate muscle regeneration.
ZIKV Replicates in Human Skeletal Muscle Progenitor Cells, Causing Cell Death
Zika virus (ZIKV) infections have raised global concerns due to severe neurological outcomes. While its neurotropic nature is well-known, the role of peripheral tissues in viral amplification is less explored. Gavino-Leooldino et al. aim to uncover the potential of skeletal muscle as an early ZIKV replication site, hypothesizing that muscle replication contributes to increased peripheral viral loads and later neural tissue invasion.
To assess whether muscle could be a ZIKV replication site, primary human skeletal muscle myoblasts (HSMM) were infected at the myoblast stage and as differentiated myotubes. Quantification of infectious particles (Fig. 1A) showed a rapid increase in ZIKV particles in myoblast cultures for at least 48 hours post-infection (hpi). Myotubes showed no increase until 16 hpi, but by 36 hpi, viral titers matched those in myoblasts. Immunostaining confirmed the presence of ZIKV E and NS2B proteins in myoblasts and myotubes at 36 hpi (Fig. 1B-E). No viral protein staining was seen in mock cultures. More ZIKV-positive cells were found in myoblast than myotube cultures at 36 and 48 hpi (Fig. 1H). Then, they assessed ZIKV replication's impact on skeletal muscle cell viability. At 48 hours post-infection (hpi), myoblast viability reduced by about 50%, whereas myotube viability remained unchanged (Fig. 1I). Consistently, myosin heavy chain (MF20) labeling showed no structural changes in myofibers of ZIKV-infected compared to mock-treated myotubes (Fig. 1F and 1G). Quantification confirmed unchanged fiber count and area (Fig. 1J), indicating ZIKV does not disrupt existing myofiber integrity. These findings suggest ZIKV replicates in myogenic cells, causing significant damage.
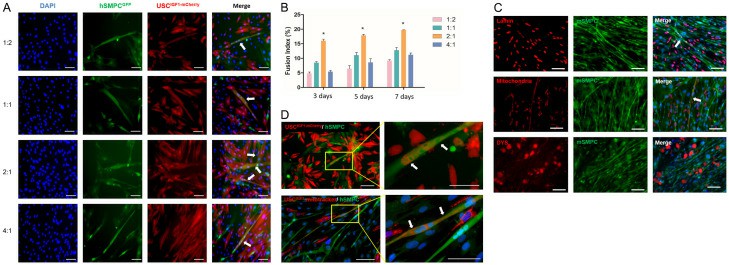 Fig. 1. ZIKV replicates and promotes death of human skeletal muscle progenitor cells.
Fig. 1. ZIKV replicates and promotes death of human skeletal muscle progenitor cells.
USCIGF1 was Fused with Human and Mouse Skeletal Muscle Progenitor Cells in vitro.
Skeletal muscles, crucial for body function, often suffer injuries affecting health and mobility. Traditional muscle regeneration relies on satellite cells, but their scarcity after severe injuries limits recovery. Stem cell therapy emerges as a promising alternative. Urine-derived stem cells (USC), easily obtained non-invasively, have shown myogenic potential.
Yi's team used USCIGF1, created by lentivirus to overexpress IGF1, assessing its effects on muscle regeneration in a rodent model. Myofusion assays were conducted between mCherry-labeled USCIGF1 and hSMPCGFP (human skeletal muscle progenitor cells labeled with a GFP-containing plasmid). The number of fused cells increased significantly, reaching 23 fused myotubes per 2×105 USCIGF1 at a 1:2 ratio after 7 days of differentiation (Fig. 2A and B). Fusion was first seen 3 days after switching to differentiation media and persisted until Day 14. Human USCIGF1 fused distinctly with mSMPCGFP, confirmed by immunostaining for human mitochondria, lamin, and dystrophin 3 (DYS3) in myotubes (Fig. 2C). In contrast, only one myotube was detectable from USC and USCmCherry controls per 2×105 cells. Cytoplasm and mitochondria were observed in myotubes with mCherry-labeled USCIGF and hSMPCGFP (Fig. 2D). These results indicated IGF1 overexpression increased the ability of USC to fuse with skeletal myocytes to form myotubes.
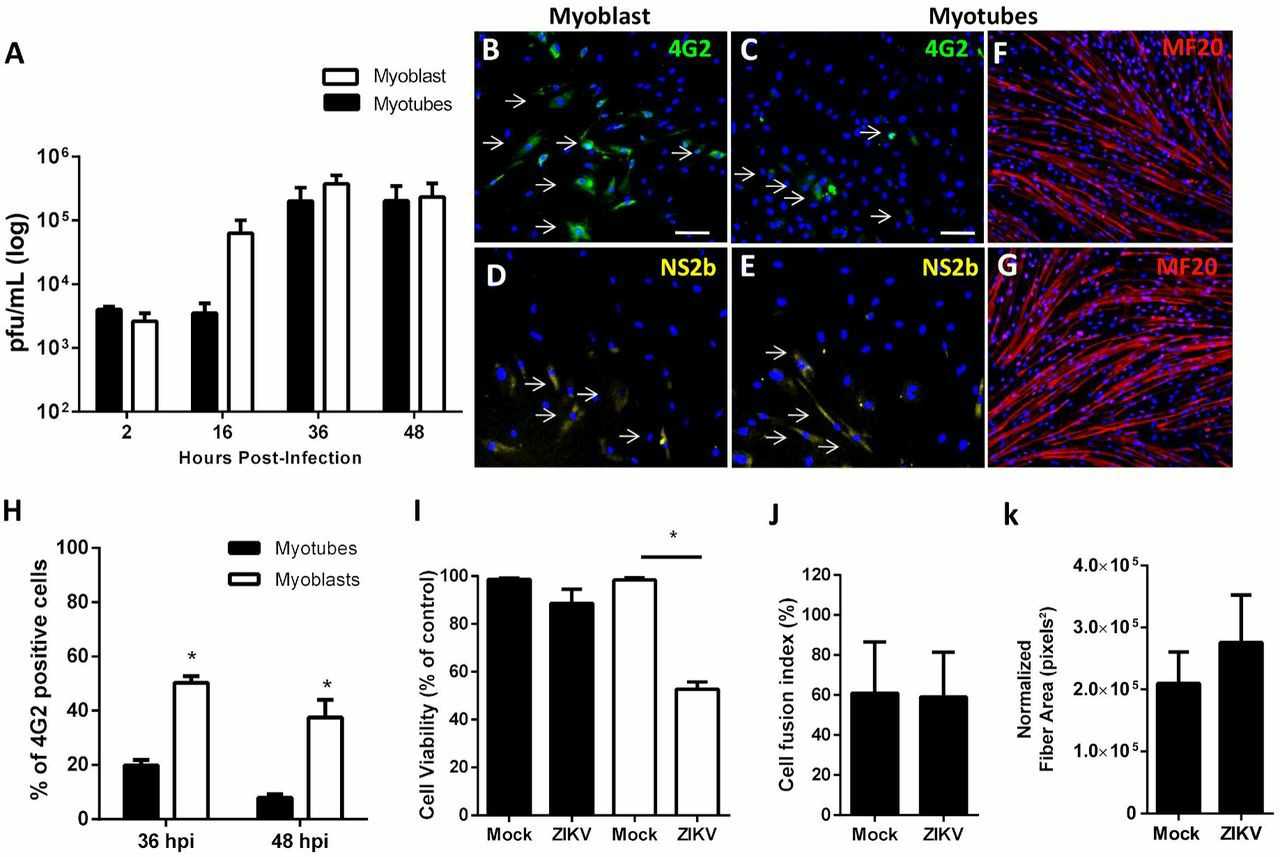 Fig. 2. USCIGF1 was fused with human skeletal muscle progenitor cells (hSMPC) and mouse skeletal muscle progenitor cells (mSMPC) in vitro (Yi H, Chen G, et al., 2024).
Fig. 2. USCIGF1 was fused with human skeletal muscle progenitor cells (hSMPC) and mouse skeletal muscle progenitor cells (mSMPC) in vitro (Yi H, Chen G, et al., 2024).
Ask a Question
Write your own review
