Human Annulus Fibrosus Cells (HAFC)
Cat.No.: CSC-7840W
Species: Human
Source: Cartilage
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
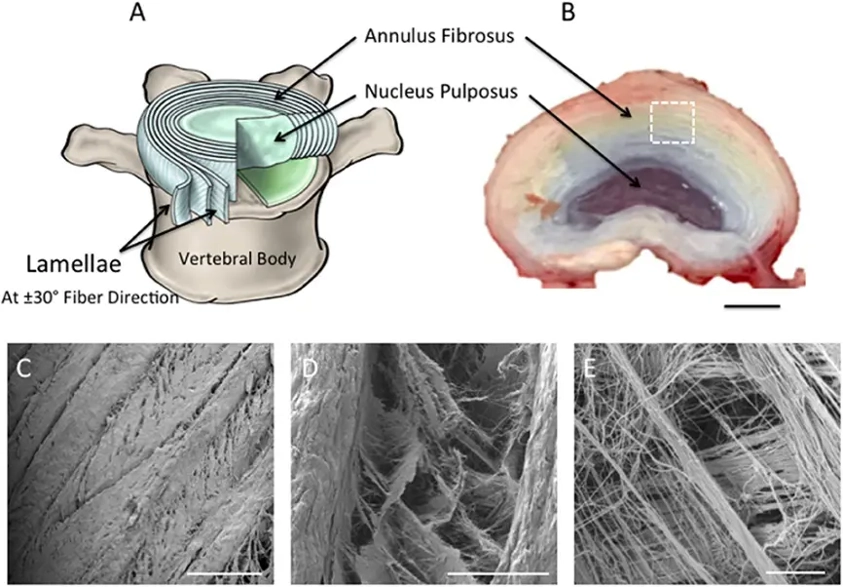
Human Annulus Fibrosus Cells (HAFC) are the primary cells that are isolated from the annulus fibrosus compartment of the intervertebral disc (IVD). The annulus fibrosus is a well-organized, fibrocartilaginous structure that surrounds the nucleus pulposus (NP). HAFC cultures maintain the morphology, phenotype, and function of native annulus fibrosus tissue, thus representing a physiologically relevant in vitro model of the tissue.
HAFC cells maintain an elongated, fibroblast-like morphology and express the extracellular matrix (ECM) components and lineage-specific markers typical for the annulus fibrosus, including types I and II collagen, aggrecan, fibronectin, and SOX9. HAFC are highly responsive to mechanical stimulation, inflammatory cytokines, and degenerative signals. Alterations in HAFC function are thought to play a direct role in IVD degeneration, which is a major cause of chronic low back pain. These cells have been extensively used in musculoskeletal and spine research to understand molecular mechanisms underlying disc development, degeneration and repair. They have also been used as an in vitro platform for evaluating potential regenerative therapies, biomaterials and anti-inflammatory or disease-modifying compounds for the treatment of IVD disorders.
mTOR and PKCδ Signaling Pathways Are Involved in HG-Induced Increase of Fibrosis Proteins
Low back pain, often caused by intervertebral disc degeneration (IVDD), is a major cause of disability. High glucose (HG) levels are implicated in IVDD, but the detailed mechanisms are unclear. Tseng et al. investigated the role of HG in promoting fibrosis in IVDD through specific signaling pathways.
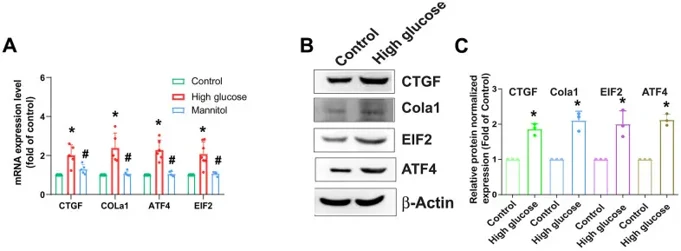
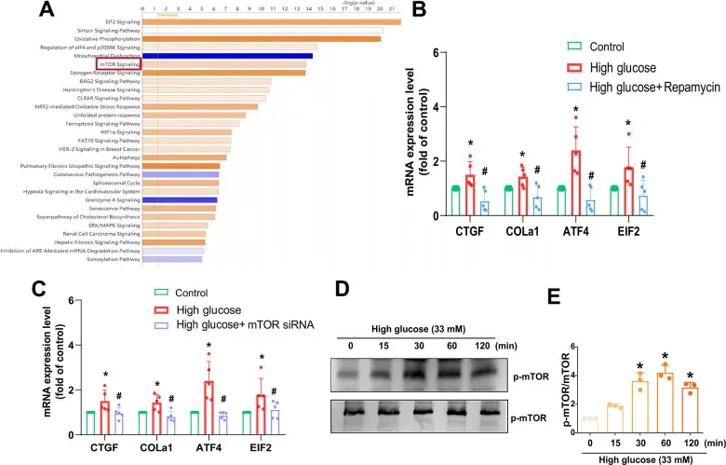
AF is crucial for IVD functionality, so HAFCs were used to study HG's effect on fibrotic protein expression. HG (33 mM) increased mRNA and protein levels of CTGF, COL1a1, ATF4, and EIF2 (Fig. 1). Osmotic controls with 33 mM mannitol showed no significant changes, indicating that HG's effect isn't due to osmolality. Using IPA software on the GSE219145 dataset, they found a significant correlation between mTOR, PKCδ, and NF-κB signaling pathways, with mTOR being the top pathway in IVDD (Fig. 2A). Pretreatment with the mTOR inhibitor Rapamycin or mTOR siRNA reduced HG-induced fibrosis protein expression (Fig. 2B, C). HG treatment of HAFCs induced time-dependent mTOR phosphorylation (Fig. 2D, E). Thus, the mTOR signaling pathway regulates HG-induced fibrosis in IVDD.
Ask a Question
Write your own review