Human Synoviocytes (HS)
Cat.No.: CSC-7708W
Species: Human
Source: Synovium
Cell Type: Fibroblast; Synoviocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human synoviocytes (HS) are mesenchymal origin cells located inside the synovial membrane. Synoviocytes display many characteristics common with fibroblasts, such as expression of several types of collagens and protein vimentin, a part of cytoskeletal filament. Once activated, synoviocytes are suggested to acquire a myofibroblast-like phenotype that drives fibrogenesis through excessive extracellular matrix component deposition and an enhanced contractile function.
Synoviocytes produce synovial fluid, are responsible for absorption from the joint cavity and are in control of blood/synovial fluid exchanges. Synoviocytes proliferate, show anchorage-independent growth, and secrete a variety of effector molecules that promote inflammation and joint destruction. Furthermore, these cells play a crucial role in the pathogenesis of chronic inflammatory diseases such as rheumatoid arthritis. Therefore, Human synoviocytes are an important model to understand articular function in degenerative and inflammatory joint diseases.
Normal human synoviocytes have been used in numerous studies, including:
- Studying changes in gene expression in synoviocytes following TNFα simulation.
- Demonstrated a role for miR-124a in the pathogenesis of arthritis.
- Demonstrated that TNF-like weak inducer of apoptosis (TWEAK) contributes to joint inflammation by inducing chemokines such as MIP-1β (CCL-4), lymphotactin (XCL-1), IFN-γ-inducible protein 10 (IP-10) (CXCL-10), MCP-1 (CCL-2), and RANTES (CCL-5), as well as the matrix metalloprotease-9, suggesting TWEAK as a novel therapeutic target.
- Showed that C/EBPβ regulates the expression of metalloproteinases and ADAMTS family members in synoviocytes stimulated with IL-1β.
- Evaluated the anti-inflammatory and antirheumatic activity of various compounds.
- Identified that cartilage link protein and MAGP2 can be used as specific markers to distinguish chondrocytes and synovial cells.
- Showed that reactive arthritis triggered by chlamydial infection is mediated by TLR2 induced IL-6 production in synoviocytes.
- Suggested that only leukocyte-poor, RBC-free platelet-rich plasma should be used in clinical orthopedics, as leukocytes and RBCs cause synoviocyte death and the production of proinflammatory mediators.
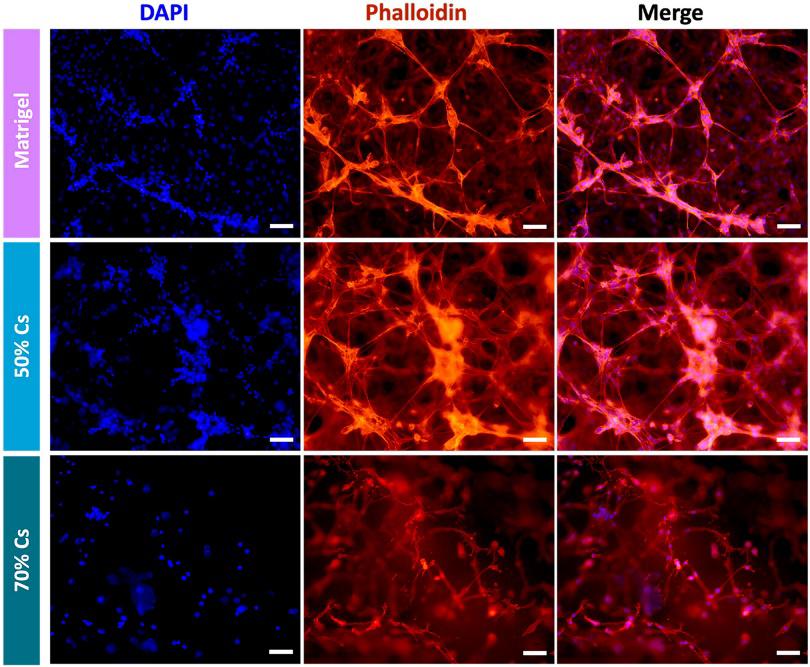
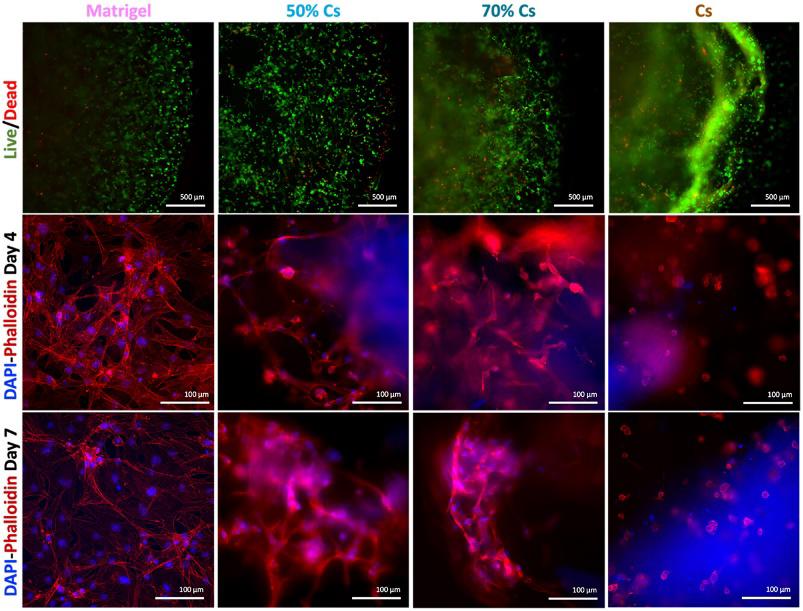
A 3D Cell Culture of Human Fibroblast-Like Synoviocytes by Encapsulation in a Chitosan-Based Hydrogel
Fibroblast-like synoviocytes (FLS), one of the cellular elements of the synovium, play a key role in rheumatoid arthritis (RA), an autoimmune disease that afflicts 1% of worldwide population. Despite articular damage starts from synovium and then proceeds involving cartilage till bone erosion, several 3D in vitro models are reported for cartilage and bone while only a few studies focused on the synovial component. Here, a hydrogel formulation suitable for 3D culturing human fibroblast-like synoviocytes and evaluated its suitability with non-RA and RA patients derived-cells is developed.
Among different formulations, a chitosan (Cs)-based hydrogel, constituted by 70% of Cs and 30% of Matrigel, showed the best results in terms of stability as well as cell growth and morphology: non-RA and RA FLS are able to grow within the hydrogel forming a complex 3D network. Thanks to the low cost, and high market availability of chitosan, this system represents a good alternative to the use of sole Matrigel for encapsulating human synoviocytes.
Ask a Question
Write your own review