Human Chondrocytes-articular (HC-a)
Cat.No.: CSC-7810W
Species: Human
Source: Cartilage
Cell Type: Chondrocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human chondrocytes are a specialized cell type found in human articular cartilage with a highly developed cytoskeleton. Chondrocytes have a round or oval shape and comprise only 5% to 10% of the total cartilage volume. These cells are primarily responsible for maintaining the biomechanical properties and extracellular matrix of cartilage. Mature chondrocytes produce essential structural proteins, including collagen types II, IX, and XI, as well as aggrecan. Articular chondrocytes also synthesize lubricin, which plays a key role in reducing friction between articulating cartilage surfaces. Since articular cartilage has a limited self-repair capacity, chondrocytes are a focus of research on cartilage degeneration and regeneration in conditions such as osteoarthritis and rheumatoid arthritis.
Studies on human chondrocytes include:
- Phenotypic characterization and differentiation into osteoclasts.
- Using chondrocytes to describe the molecular biology of cell receptors, signaling cascades, cytokine activation and gene regulation.
- The cells have been implicated in apoptosis, cytotoxicity, and cartilage degradation seen in inflammatory diseases such as rheumatoid arthritis, osteoarthritis and Lyme disease-associated arthritis.
- By examining the effects of shear stress and mechanotransduction pathways, some hope to develop treatments that can prevent erosive joint pathology.
- Some labs are focusing on monoclonal antibody therapy, or inhibition of erosive matrix metalloproteinases for the treatment of arthritis.
Expansion and Delivery of Human Chondrocytes on Gelatin-Based Cell Carriers
Autologous chondrocyte implantation (ACI) is a widely used cell-based therapy for repairing cartilage damage in clinical practice. However, in vitro expansion of chondrocytes on standard 2D culture surfaces leads to dedifferentiation (loss of the chondrocyte phenotype), and the delivery of detached cells has proven to be ineffective. To overcome these limitations, the matrix-assisted ACI (MACI) procedure was developed. In MACI, matrices such as hydrogels and microspheres are used as cell carriers or scaffolds to deliver expanded chondrocytes, enhancing cell viability and precision delivery.
This study aims to investigate various configurations of gelatin-based hydrogels for their potential to support both cell expansion and delivery as a single step. This study evaluated gelatin microspheres (Gel MS), micronized photo-crosslinked GelMA microparticles (GelMA MP), and bulky GelMA hydrogels containing cells (GelMA HG). The results demonstrate that normal human knee articular chondrocytes exhibit robust growth on Gel MS and form aggregates enriched with glycosaminoglycan-rich extracellular matrix (ECM). Gel MS outperformed both GelMA MP and GelMA HG as a cell carrier by both supporting long-term cell growth with reduced dedifferentiation and precision delivery.
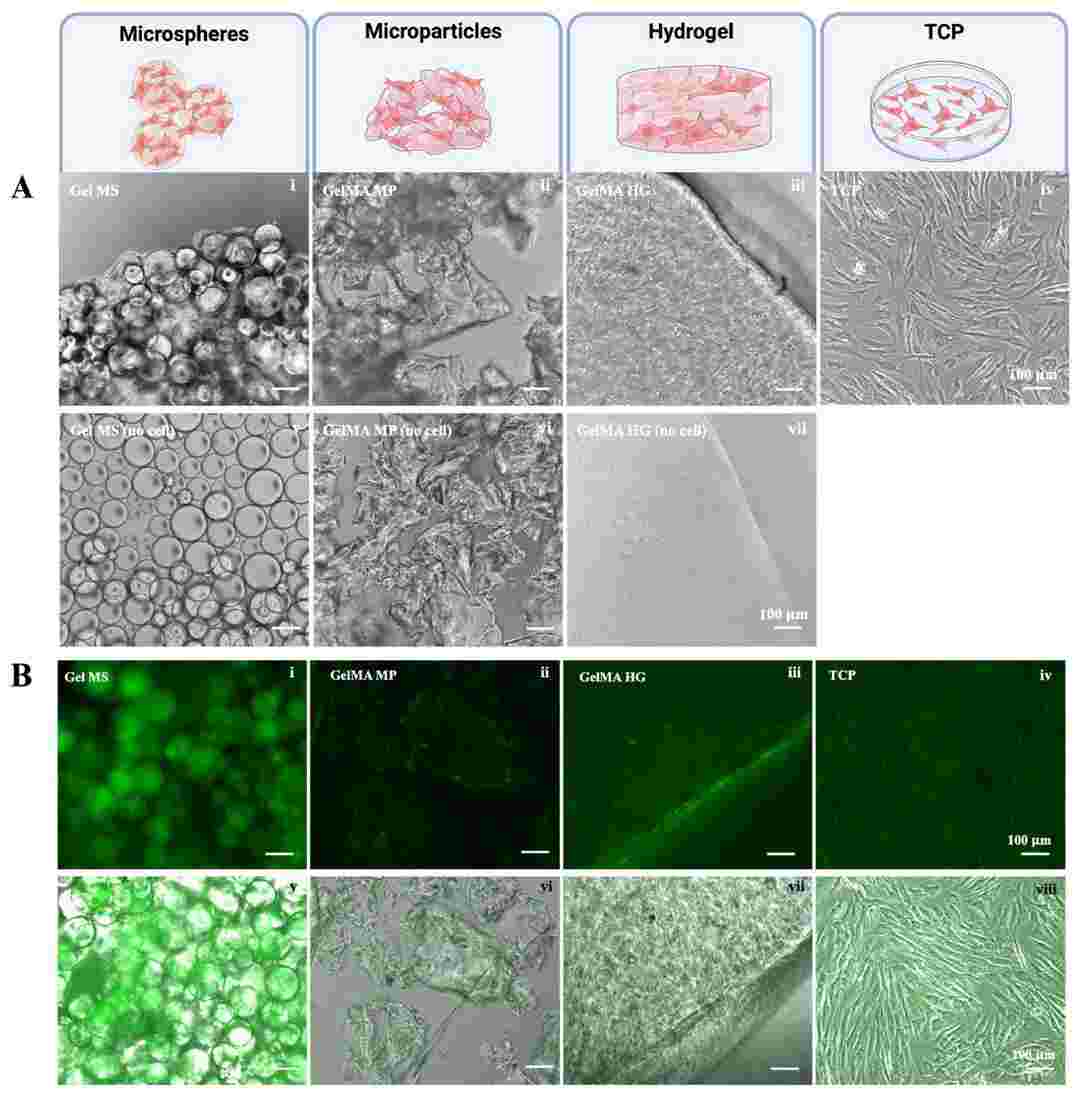 Fig. 1. Culturing chondrocytes on Gel MSs (i), GelMA MPs (ii), GelMA HGs (iii), or on TCPs (iv) (Patel, Krishi, Derya Ozhava, and Yong Mao. 2025).
Fig. 1. Culturing chondrocytes on Gel MSs (i), GelMA MPs (ii), GelMA HGs (iii), or on TCPs (iv) (Patel, Krishi, Derya Ozhava, and Yong Mao. 2025).
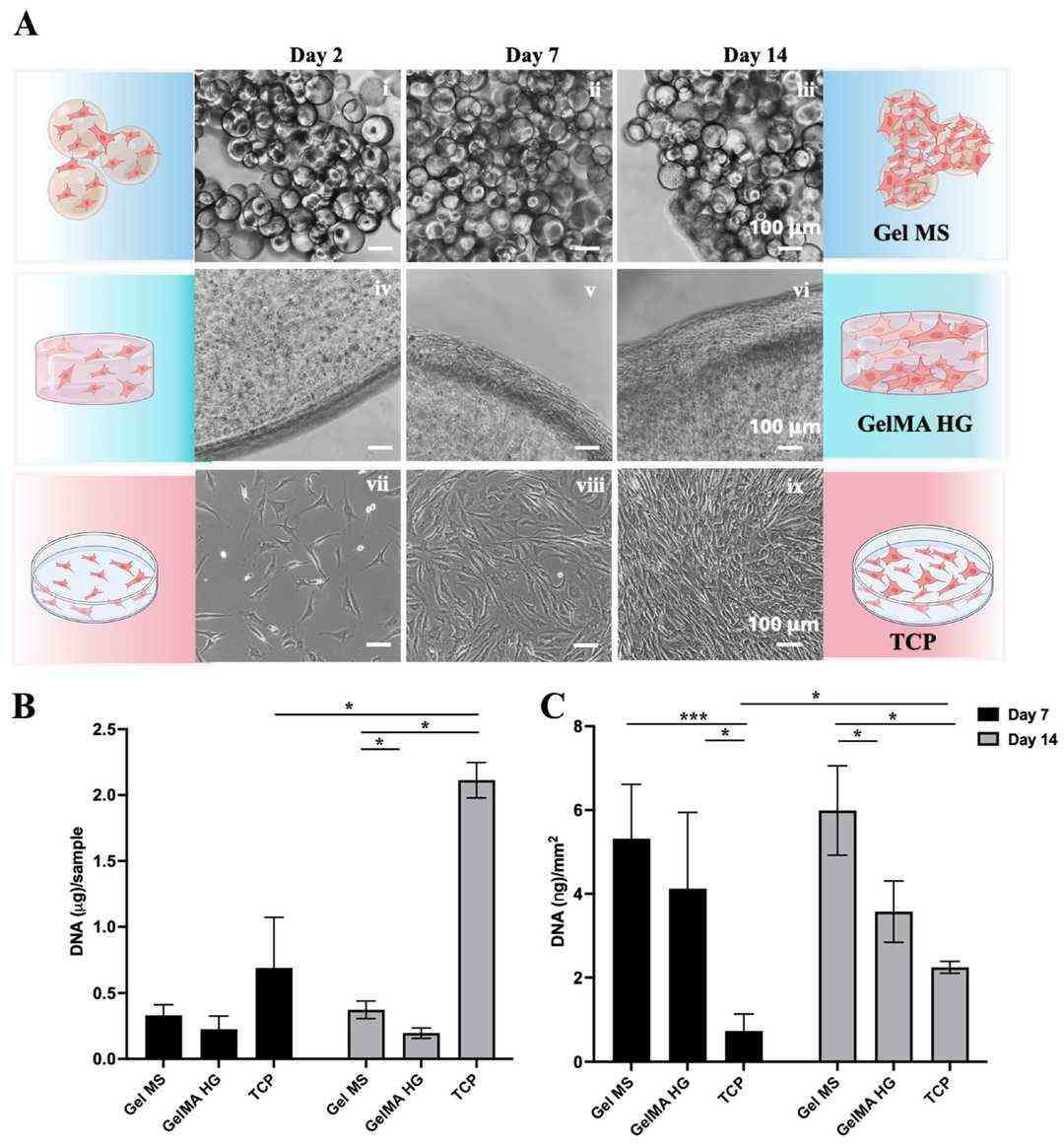 Fig. 2. Cell expansion on Gel MSs, GelMA HGs, or TCPs (Patel, Krishi, Derya Ozhava, and Yong Mao. 2025).
Fig. 2. Cell expansion on Gel MSs, GelMA HGs, or TCPs (Patel, Krishi, Derya Ozhava, and Yong Mao. 2025).
Benzophenone-3 Exposure Induced Apoptosis in Human Chondrocytes
Osteoarthritis (OA) is a chronic joint disease affecting millions of adults worldwide, characterized by the degeneration of articular cartilage. Benzophenone-3 (BP-3), a commonly used ultraviolet filter in personal care products, has been positively associated with OA risk. However, it remains unclear whether and how BP-3 induces toxic effects on articular chondrocytes and promote OA development. This study aims to investigate the damage of BP-3 at environmentally relevant concentrations to human chondrocytes, as well as potential mechanisms linking BP-3 with injury of chondrocytes.
In brief, BP-3 exposure may increase OA risk via inducing apoptosis and increasing breakdown of extracellular matrix in chondrocytes, and mitochondrial dysfunction and mitophagy may play a crucial role in the mechanisms of BP-3-induced toxicity to articular chondrocytes.
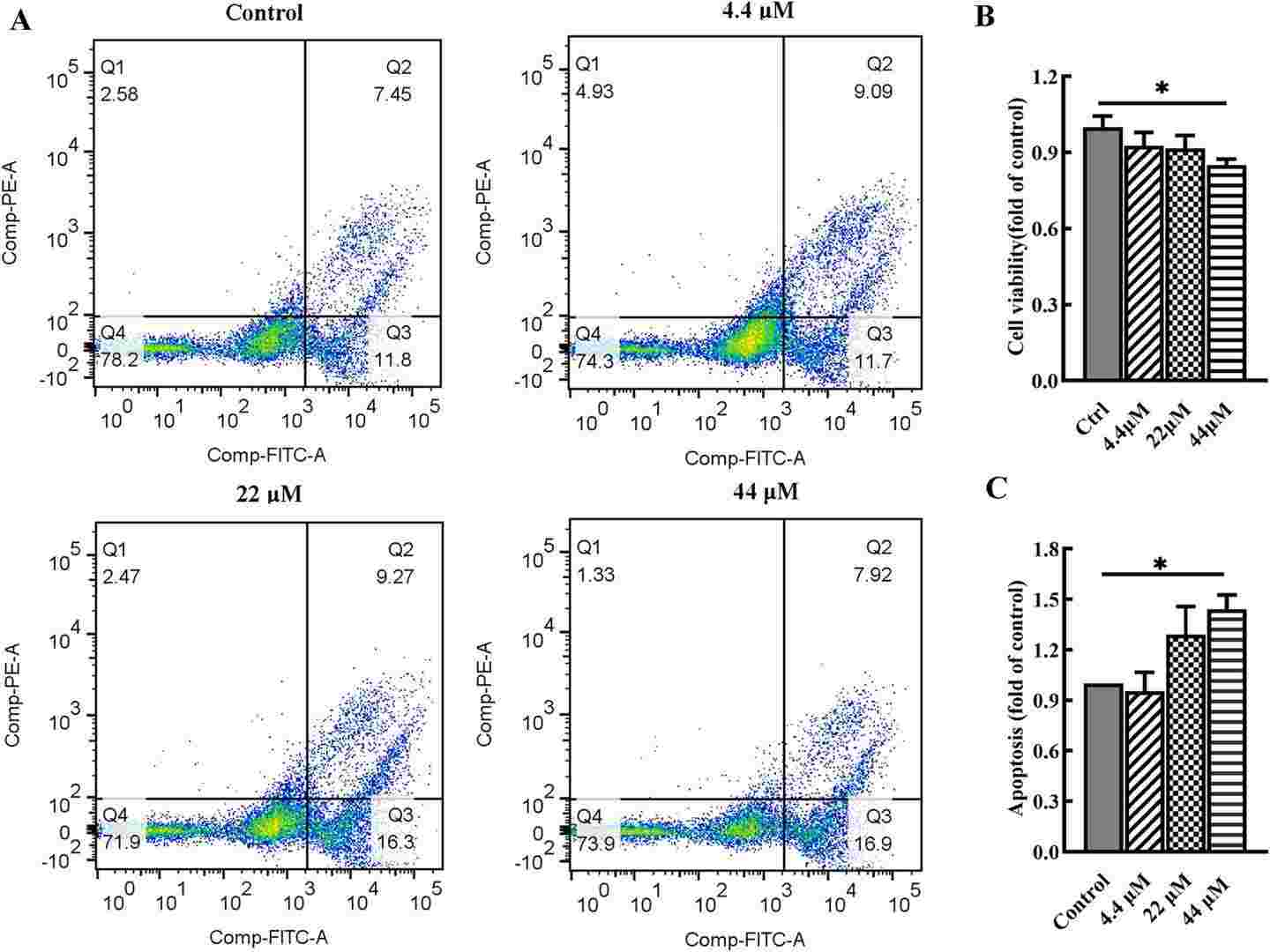 Fig. 3. Effects of BP-3 exposure on cell viability and apoptosis in chondrocytes (Yang, Ye, et al. 2024).
Fig. 3. Effects of BP-3 exposure on cell viability and apoptosis in chondrocytes (Yang, Ye, et al. 2024).
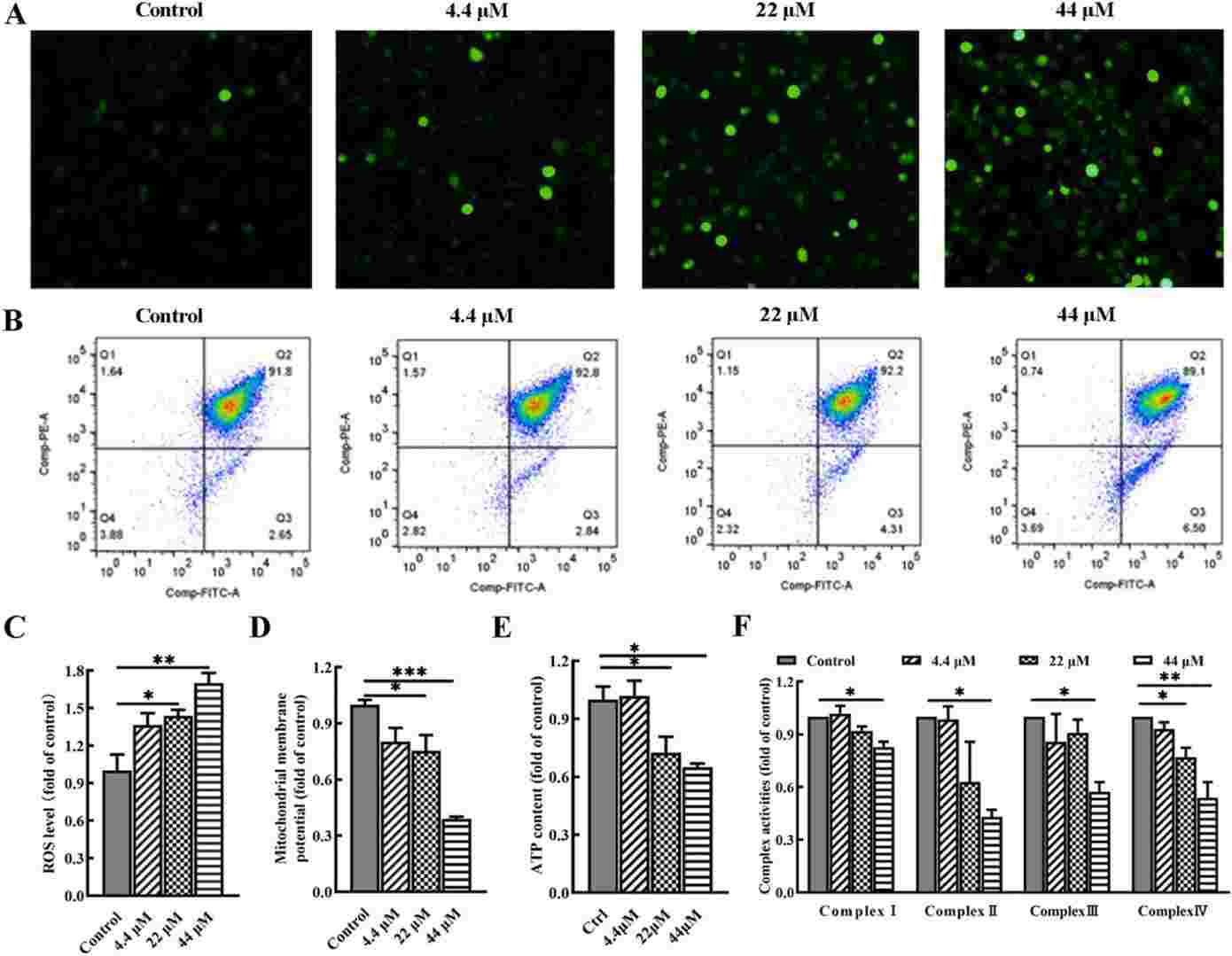 Fig. 4. BP-3 exposure disrupts mitochondrial function in chondrocytes (Yang, Ye, et al. 2024).
Fig. 4. BP-3 exposure disrupts mitochondrial function in chondrocytes (Yang, Ye, et al. 2024).
Ask a Question
Write your own review