Human Pancreatic Stellate Cells (HPaSteC)
Cat.No.: CSC-7740W
Species: Human
Source: Pancreas
Cell Type: Pancreatic Stellate Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Pancreatic Stellate Cells (PSCs) are located adjacent to the basolateral aspect of pancreatic acinar cells and have also been identified around small pancreatic ducts and blood vessels. They account for approximately 4-7% of the total cell mass in the gland. In a healthy pancreas, PSCs exhibit abundant, vitamin A containing lipid droplets in their cytoplasm and are in a quiescent (non-activated) state. They can be distinguished from fibroblasts by the expression of selective markers such as desmin, glial fibrillary acidic protein (GFAP), vimentin and nestin (intermediate filament proteins) and neuroectodermal markers such as nerve growth factor (NGF) and neural cell adhesion molecule (NCAM). On electron microscopic examination, PSCs reveal a prominent rough endoplasmic reticulum, collagen fibrils and vacuoles (lipid droplets) surrounding a central nucleus.
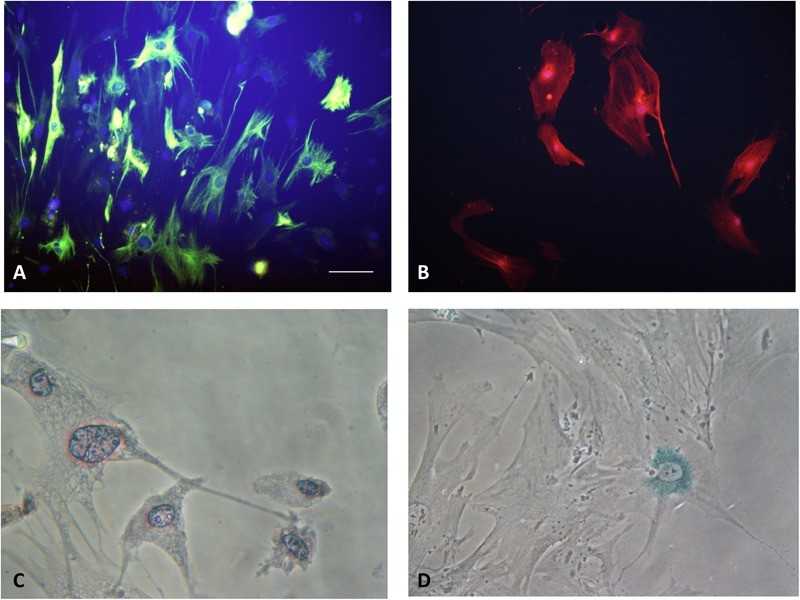 Fig. 1. The Type of PSCs (Ran Xue, et al. 2018). (A) Quiescent PSCs stain positively for desmin. (B) Activated PSCs stain positively for α-SMA. (C) Quiescent PSCs have an angular appearance, contained lipid droplets by oil red O staining. (D) Senescence is shown in PSCs with cytoplasmic blue staining of SA-β-gal.
Fig. 1. The Type of PSCs (Ran Xue, et al. 2018). (A) Quiescent PSCs stain positively for desmin. (B) Activated PSCs stain positively for α-SMA. (C) Quiescent PSCs have an angular appearance, contained lipid droplets by oil red O staining. (D) Senescence is shown in PSCs with cytoplasmic blue staining of SA-β-gal.
PSCs are major producers of extracellular matrix (ECM) proteins such as collagen I-IV, fibronectin, and laminin. They also produce ECM degrading enzymes like matrix metalloproteinases (MMPs) and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMPs). In the normal pancreas, these enzymes maintain an balance between the deposition and degradation of ECM proteins. PSCs also play an important role in innate immunity by expressing Toll-like receptors (TLRs) 2, 3, 4 and 5, which can recognize pathogen-associated molecular patterns (PAMPs), alarmins and endogenous molecules released by tissue damage (DAMPs). PSCs may also possess progenitor-like properties, as they express multiple stem cell markers, including CD133, SOX9, nestin, and GDF3. They can differentiate into other cell types including insulin-secreting cells under the influence of relevant growth factors.
Human Pancreatic Stellate Cells (HPaSteC) from Creative Bioarray are isolated from human pancreas. HPaSteC are cryopreserved at passage one and delivered frozen. Each vial contains more than 5 x 105 cells. HPaSteC are characterized by immunofluorescence with antibodies specific to α-smooth muscle actin. HPaSteC are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. Creative Bioarray also provides optimized media and reagents for the growth of human pancreatic stellate cells, which are quality tested together and guaranteed to give maximum performance as a global solution for in vitro HPaSteC culture.
Pancreatic Stellate Cells Support Human Pancreatic β-Cell Viability In Vitro and Enhance Survival of Human Islets Exposed to Cytokines
Pancreatic islet transplantation is proposed as a cure for type 1 diabetes mellitus (T1D). Inflammatory stress post-transplantation and loss of extracellular matrix attribute to the limited β-cell survival. Pancreatic stellate cells (PSCs), identified as pancreatic-specific stromal cells, have the potential to play a crucial role in preserving islet survival. Transwell culture inserts were utilized to assess the paracrine impact of PSCs on β-cells, alongside co-cultures enabling direct interaction between PSCs and human islets. We found that co-culturing PSCs with human CM β-cells and human cadaveric islets had rescuing effects on cytokine-induced stress. PSCs were associated with upregulation of β-cell mitochondrial activity and suppression of inflammatory gene expression. The rescuing effects exist both in indirect and direct co-culture methods. Our findings show novel effects of PSCs on islet health. Islets and PSCs coculturing or co-transplantation might mitigate the inflammation stress and improve islet transplantation outcomes.
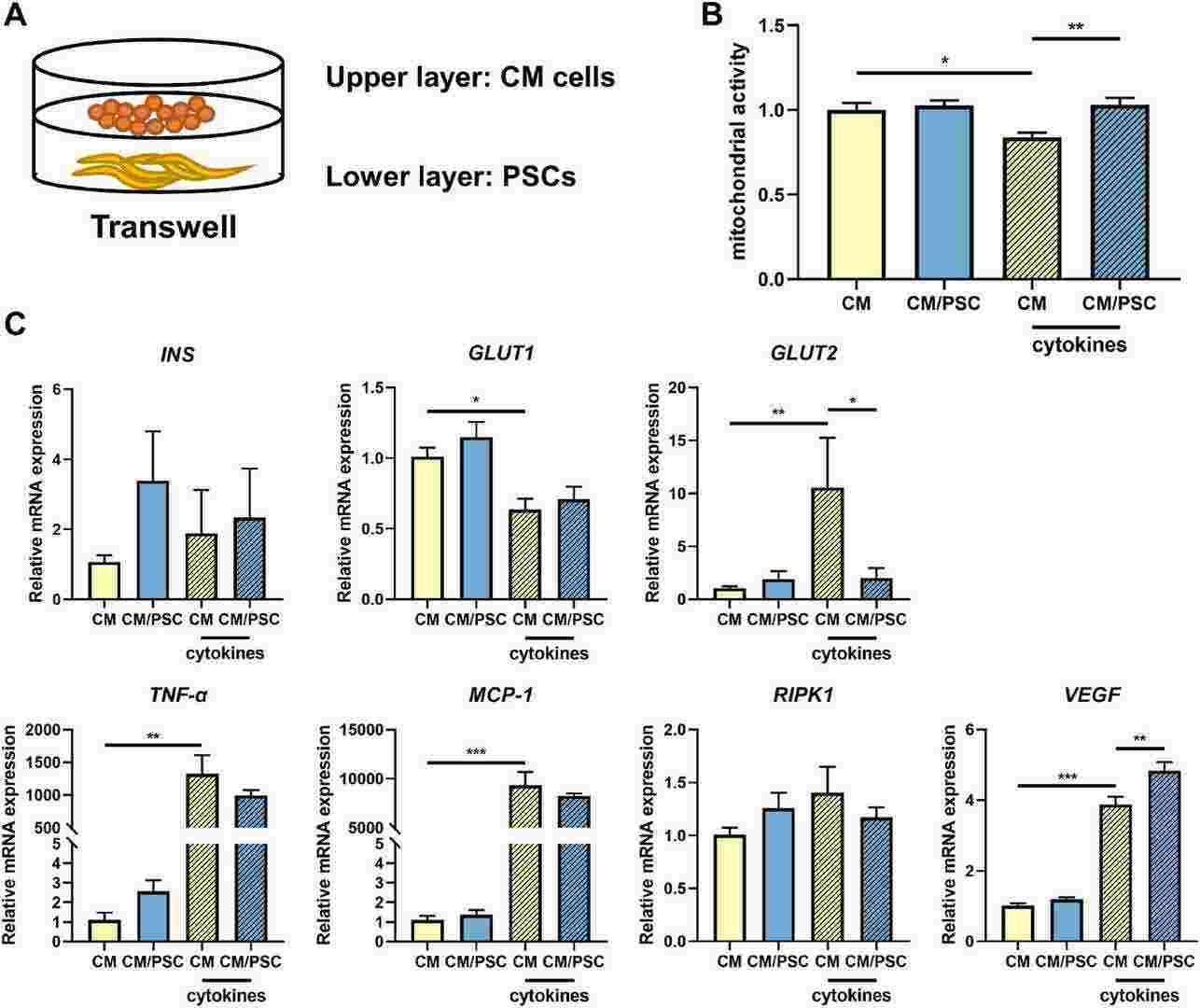 Fig. 1. Mitochondrial activity and gene expression levels in CM β-cells in presence and absence of the cytokine mix of IFN-γ, TNF-α and IL-1β (Qin, Tian, et al. 2024).
Fig. 1. Mitochondrial activity and gene expression levels in CM β-cells in presence and absence of the cytokine mix of IFN-γ, TNF-α and IL-1β (Qin, Tian, et al. 2024).
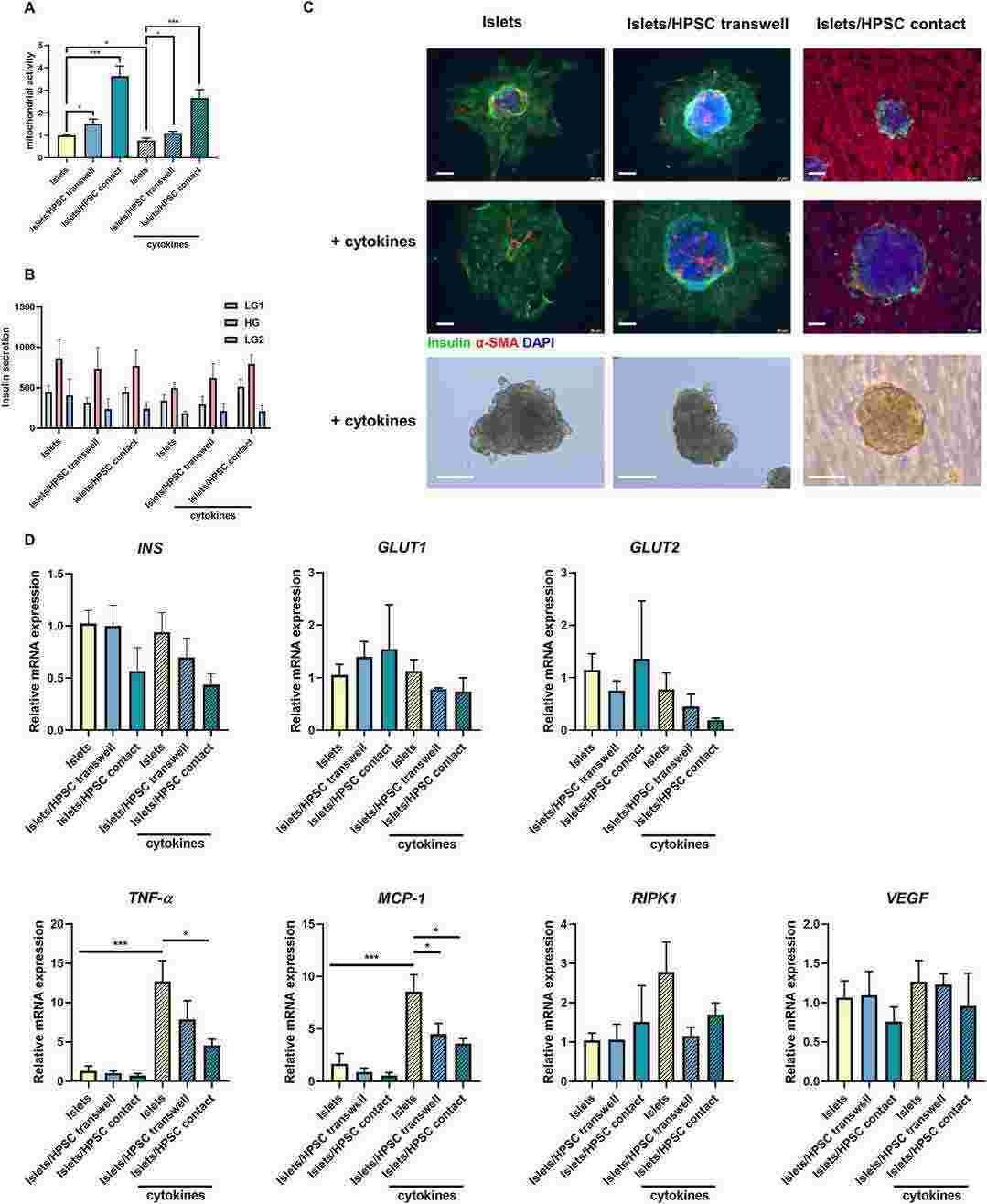 Fig. 2. The co-culture of HPSCs preserved human islet mitochondrial activity in vitro in presence and absence of IFN-γ, TNF-α and IL-1β (Qin, Tian, et al. 2024).
Fig. 2. The co-culture of HPSCs preserved human islet mitochondrial activity in vitro in presence and absence of IFN-γ, TNF-α and IL-1β (Qin, Tian, et al. 2024).
Disruption of Super-Enhancers in Activated Pancreatic Stellate Cells Facilitates Chemotherapy and Immunotherapy in Pancreatic Cancer
A major obstacle in the drug treatment of pancreatic ductal adenocarcinoma (PDAC) is its highly fibrotic tumor microenvironment, which is filled with activated pancreatic stellate cells (a-PSCs). These a-PSCs generate a large amount of extracellular matrix and secrete various cytokines, forming biophysical and biochemical barriers that hinder drug access to tumor tissues. Therefore, it is imperative to develop a strategy for reversing PSC activation and thereby eliminate the barriers to facilitate PDAC drug treatment. Herein, by integrating chromatin immunoprecipitation (ChIP)-seq, Assays for Transposase-Accessible Chromatin (ATAC)-seq, and RNA-seq techniques, this work reveals that super-enhancers (SEs) promote the expression of various genes involved in PSC activation. Disruption of SE-associated transcription with JQ1 reverses the activated phenotype of a-PSCs and decreases stromal fibrosis in both orthotopic and patient-derived xenograft (PDX) models. In summary, this study not only elucidates the contribution of SEs of a-PSCs in shaping the PDAC tumor microenvironment but also highlights that targeting SEs in a-PSCs may become a gate-opening strategy that benefits PDAC drug therapy.
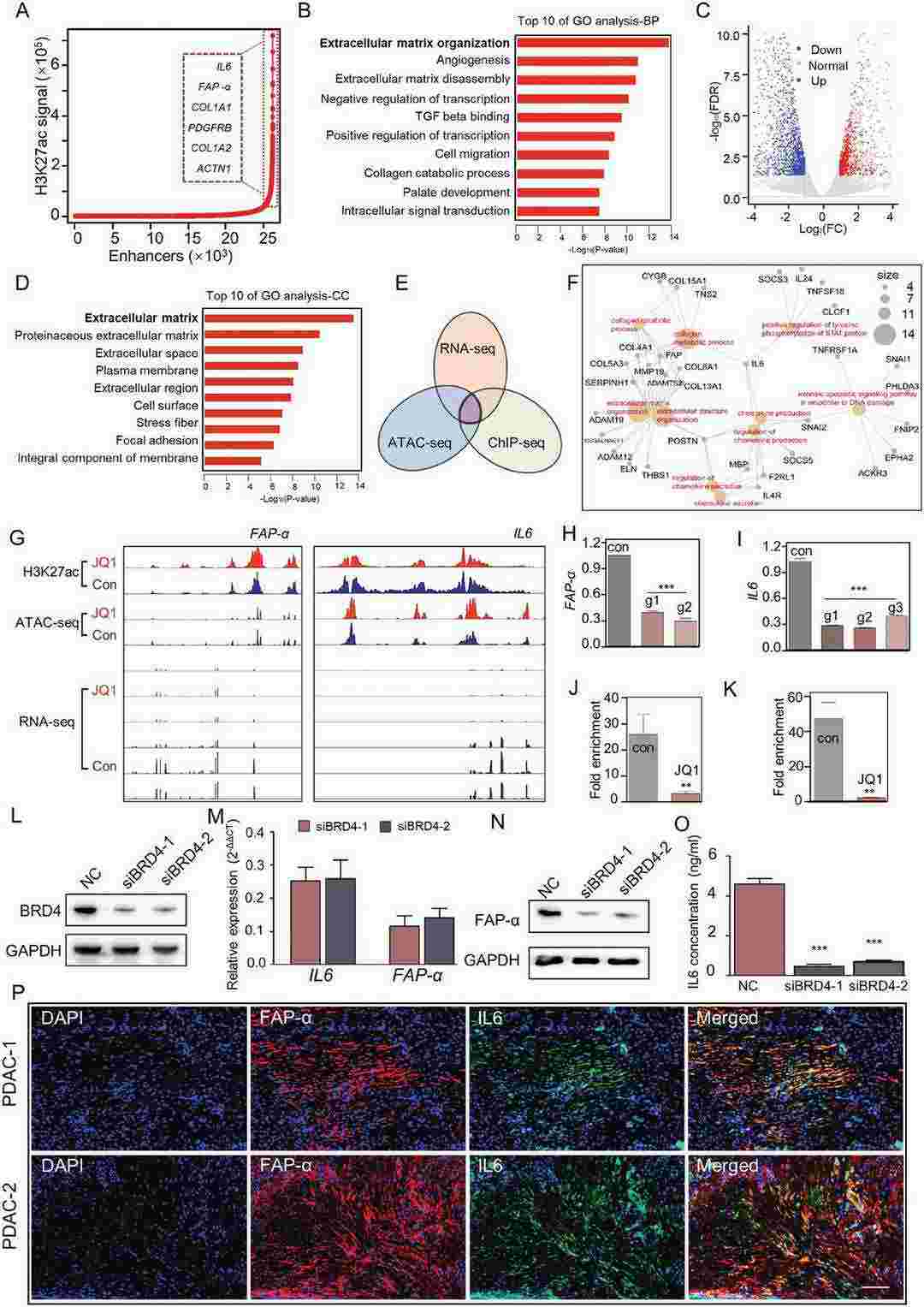 Fig. 3. Super-enhancers (SEs) were associated with genes involved in a-PSCs activation (Wang, Yazhou, et al. 2024).
Fig. 3. Super-enhancers (SEs) were associated with genes involved in a-PSCs activation (Wang, Yazhou, et al. 2024).
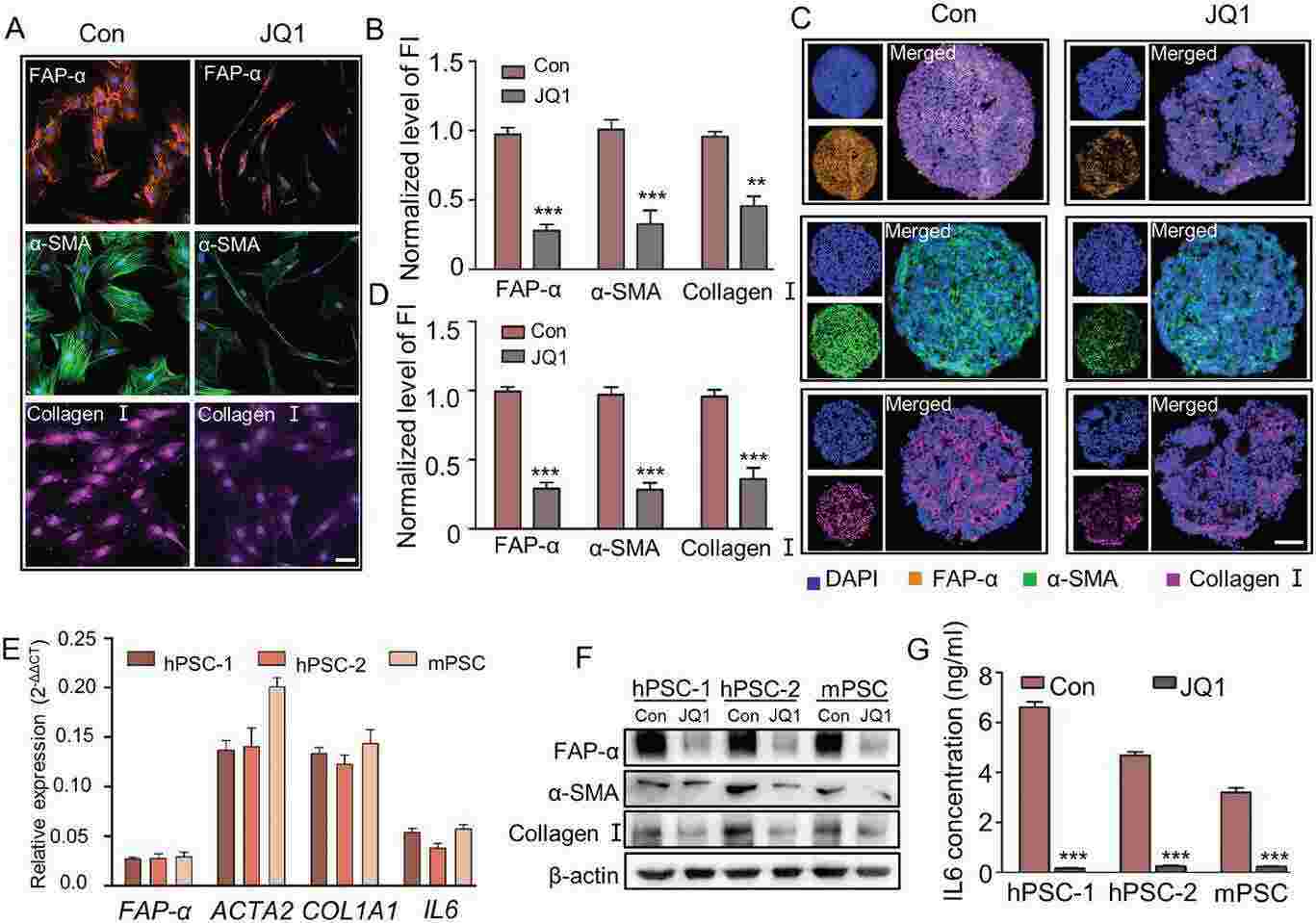 Fig. 4. JQ1 treatment reversed the activated phenotype of Human a-PSCs at both mRNA and protein levels (Wang, Yazhou, et al. 2024).
Fig. 4. JQ1 treatment reversed the activated phenotype of Human a-PSCs at both mRNA and protein levels (Wang, Yazhou, et al. 2024).
Ask a Question
Write your own review