Human Valvular Interstitial Cells
Cat.No.: CSC-7849W
Species: Human
Source: Heart
Cell Type: Interstitial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Development period: Prenata
Human Valvular Interstitial Cells (HVICs) are the most abundant cell type found in cardiac valves and are widely distributed in the aortic valve, pulmonary valve, tricuspid valve, and mitral valve. They are present on both the outer (aortic surface) and inner (endocardial surface) sides of the valve leaflets, although their distribution and functional properties may vary across different regions. HVICs have the ability to synthesize and secrete various extracellular matrix (ECM) components, including collagen, elastin, and proteoglycans. They play a role in regulating valve remodeling by secreting matrix metalloproteinases (MMPs) and cathepsins (such as cathepsin S and K) to degrade the ECM. In calcific aortic valve disease (CAVD), HVICs can undergo a transformation into osteoblastic valve interstitial cells (osteoblastic VICs) and secrete bone formation-related proteins, such as alkaline phosphatase and osteocalcin, leading to the formation of calcified nodules within the valve. TGF-β1 and biglycan (a lipid that accumulates in CAVD lesions) are suggested to be major mediators of HVIC calcification. Additionally, HVICs are capable of secreting pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), promoting the formation of new blood vessels, which contributes to the pathogenesis of valve disease. Under inflammatory conditions, HVICs can secrete a variety of cytokines (e.g., IL-1β), thus participating in inflammatory responses and immune regulation.
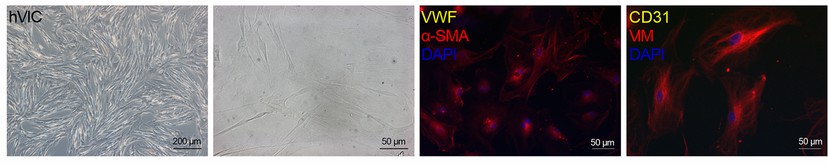 Fig. 1. Brightfield images and immunofluorescence staining of hVICs showing positive staining for alpha smooth muscle actin (α-SMA) and vimentin (VIM) (Nehl D, Goody P R, et al., 2023).
Fig. 1. Brightfield images and immunofluorescence staining of hVICs showing positive staining for alpha smooth muscle actin (α-SMA) and vimentin (VIM) (Nehl D, Goody P R, et al., 2023).
Indoxyl Sulfate Accentuates the OM-Induced Osteogenic Transition and Calcification of hVICs
Calcific aortic stenosis is accelerated in chronic kidney disease, yet no therapy exists. The uremic toxin indoxyl sulfate (IS) strongly predicts cardiovascular death in CKD and promotes ectopic calcification, but its role in aortic valve mineralization is unknown. Candellier et al. therefore asked whether IS drives human valvular interstitial cells (hVICs) toward an osteogenic, calcifying phenotype and identified the underlying mechanisms.
To evaluate the effects of IS on hVIC osteogenic transition and mineralization, hVICs were exposed to ISn (2 μmol/l), ISu (148 μmol/l) or ISmax (934 μmol/l) in the presence of an OM. As presented in Figure 1, a 3-day exposure to the OM promoted hVICs osteogenic transition, evidenced by the elevation of the expression of the main osteogenic markers BMP2 and RUNX2 both at the mRNA (Fig. 1A and B) and protein (Fig. 1C-E) level. Exposure to this OM promoted hVIC mineralization within 14 days (Fig. 1F-H). Interestingly, a 3-day exposure to ISu or ISmax promoted a 2-fold increase in OM-induced mRNA expression of BMP2 (Fig. 2A) and RUNX2 (Fig. 2B), relative to cells cultured in the absence of IS (control (CT) condition, represented by a dotted line in the graphs). If this increased osteogenic transition was not sufficient to promote OM-induced calcification after only 3 days of incubation, a 14-day exposure to IS increased OM-induced hVIC mineralization in a concentration-dependent manner, relative to CT cells (ISu: +46% vs. CT (p<0.05), ISmax: +153% vs. CT (p<0.05)) (Fig. 2C and D). In contrast, treatment with ISn had no impact on OM-induced expression of BMP2 or RUNX2 or OM-driven mineralization, relative to CT cells (Fig. 2A-D).
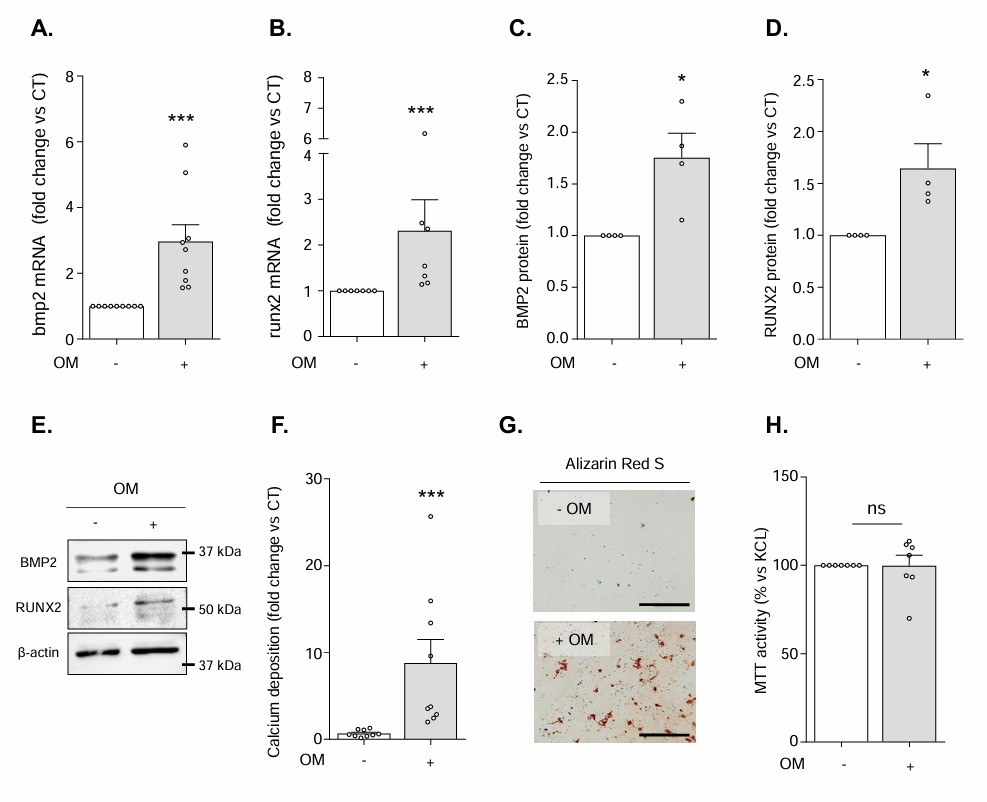 Fig. 1. Exposure to the osteogenic medium (OM) promotes hVICs osteogenic transition and subsequent mineralization without modulation of cell viability (Candellier A, Issa N, et al., 2023).
Fig. 1. Exposure to the osteogenic medium (OM) promotes hVICs osteogenic transition and subsequent mineralization without modulation of cell viability (Candellier A, Issa N, et al., 2023).
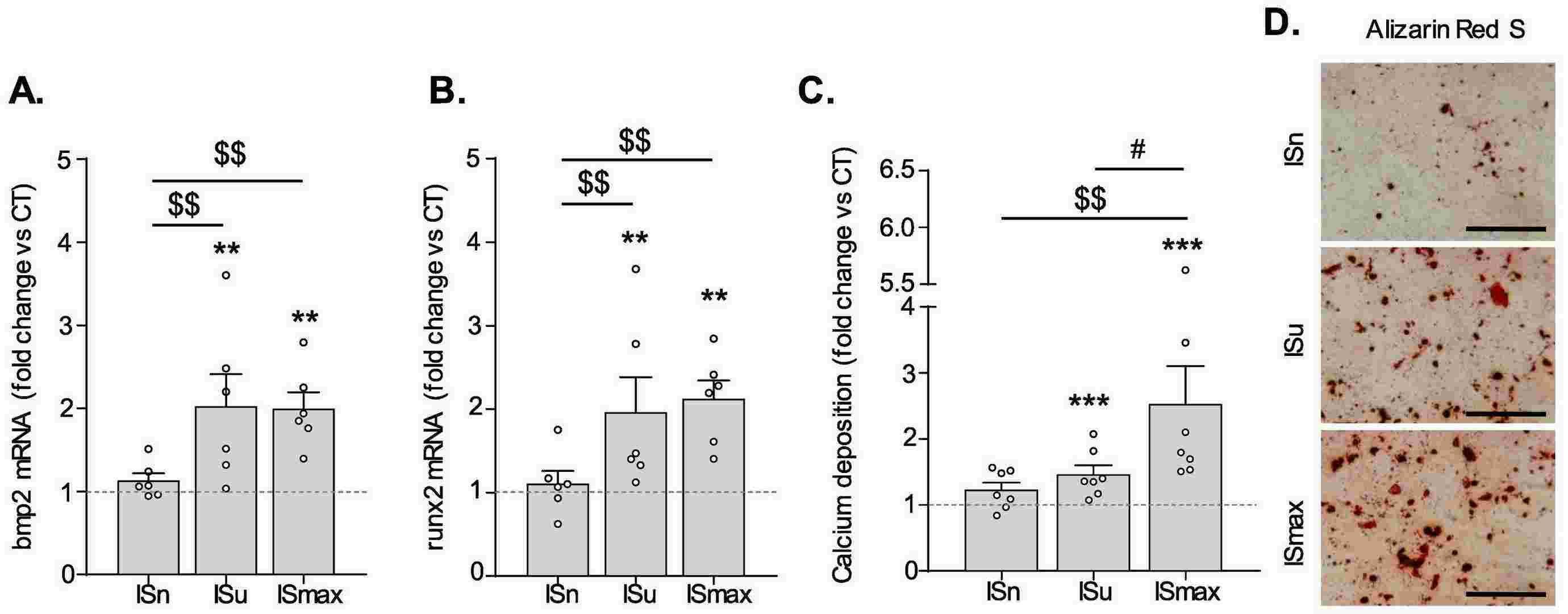 Fig. 2. Indoxyl-sulfate promotes OM-induced hVICs osteogenic transition and subsequent mineralization (Candellier A, Issa N, et al., 2023).
Fig. 2. Indoxyl-sulfate promotes OM-induced hVICs osteogenic transition and subsequent mineralization (Candellier A, Issa N, et al., 2023).
DUSP26 Interacts with DPP4 and Prevents its Degradation in hVICs
Calcific aortic valve disease (CAVD) is a major health concern owing to its high morbidity, mortality, and lack of available treatments. Wang’s team utilized human calcific aortic valves and normal control microarray profiling, ApoE−/− mouse models, and human valvular interstitial cells (hVICs) to study the role of DUSP26 in CAVD with the goal of identifying new therapeutic targets.
HVICs immunoprecipitated with IgG or anti-DUSP26 antibody (Fig. 3A) were analyzed by mass spectrometry, which revealed DPP4 as a possible DUSP26-binding protein in hVICs (Fig. 3B). To verify, hVICs were co-transfected with HA-tagged DPP4 and Flag-tagged DUSP26 (Fig. 3C). Results of co-IP confirmed that the overexpressed Flag-DUSP26 binds to HA-DPP4 in hVICs (Fig. 3C), and endogenous DUSP26/DPP4 protein interaction was also confirmed (Fig. 3D). As DUSP26 is a phosphatase, it was determined whether phosphatase activity was required for DPP4 binding. No difference in binding was found in hVICs when treated with the DUSP26 inhibitor F1063-0967 (Fig. 3E). Additionally, the phosphatase-dead mutant (C152S) did not lose binding ability (Fig. 3F). Thus, DUSP26 does not require phosphatase activity to bind to DPP4. Next, plasmids of DPP4-N-terminal (amino acids 1–324) and DPP4-C-terminal (amino acids 325–766) were constructed to determine the interacting region (Fig. 3G). In vitro translation and IP analyses revealed that the DPP4 C-terminus interacts with DUSP26 (Fig. 3H), which reveals that DUSP26 binds to the C-terminus of DPP4.
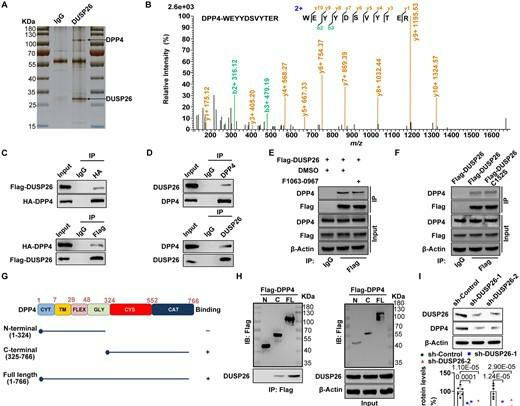 Fig. 3. DUSP26 interacts with DPP4 and protects it from ubiquitin-mediated degradation in hVICs (Wang Y J, Han D, et al., 2021).
Fig. 3. DUSP26 interacts with DPP4 and protects it from ubiquitin-mediated degradation in hVICs (Wang Y J, Han D, et al., 2021).
Ask a Question
Write your own review