Human Renal Proximal Tubular Epithelial Cells (HRPTEpiC)
Cat.No.: CSC-7718W
Species: Human
Source: Kidney
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The human renal proximal tubular epithelial cells (HRPTEpiC) derive from the kidney's proximal tubules that perform crucial roles in substance reabsorption and secretion. The renal tubule begins with cells connected to the glomerulus that reabsorb water and electrolytes while also recovering glucose and managing urine concentration and acidity. HRPTEpiC maintain a cuboidal or columnar morphology in vitro which matches the typical appearance of tubular epithelial cells. These cells display a unique basement membrane structure and express specific epithelial markers including Laminin and the apical membrane transporter SGLT2. Throughout the culture process these cells maintain their polarized distribution which results in a distinct separation between their apical and basal membranes.
HRPTEpiC serve essential functions in multiple research areas. HRPTEpiC serve as research tools to investigate various kidney diseases including diabetic nephropathy along with HIV-associated nephropathy (HIVAN) and BK virus infection. Three-dimensional culture techniques enable HRPTEpiC to replicate kidney development processes which introduces new research tools for studying kidney development. The reactions of these cells to nicotine and HIV infection provide essential information regarding how renal toxicity functions.
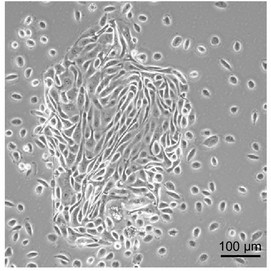 Fig. 1. Primary renal proximal tubular epithelial cells (RPTECs) (Jun DY, Kim YS, et al., 2018).
Fig. 1. Primary renal proximal tubular epithelial cells (RPTECs) (Jun DY, Kim YS, et al., 2018).
A Unifying Model of Glucotoxicity in Human Renal Proximal Tubular Epithelial Cells and the Effect of the SGLT2 Inhibitor Dapagliflozin
Diabetic nephropathy, a leading cause of end-stage renal disease, is closely linked to glucotoxicity in human renal proximal tubular epithelial cells (RPTECs). Sodium-glucose cotransporter 2 (SGLT2) inhibitors have shown renoprotective effects, possibly due to mitigating glucotoxicity rather than merely controlling glucose levels. Elefthriadis et al. aims to propose a unified model of glucotoxicity previously observed in capillary endothelial cells for RPTECs.
They first assessed the impact of elevated glucose on RPTECs' glucose uptake and dapagliflozin's effect. RPTECs show increased glucose uptake under high glucose conditions compared to normal. Dapagliflozin reduces glucose consumption in both settings, but high glucose levels remain elevated compared to normal glucose conditions (Fig. 1a). They also examined SGLT2 expression: high glucose induces its expression, while dapagliflozin reduces SGLT2 levels in both conditions (Fig. 1b, c). We evaluated ROS production, finding it enhanced under high glucose, and dapagliflozin mitigates this increase (Fig. 2a). Additionally, oxidative stress inhibits GAPDH activity in high glucose conditions, but dapagliflozin restores it to normal levels (Fig. 2b).
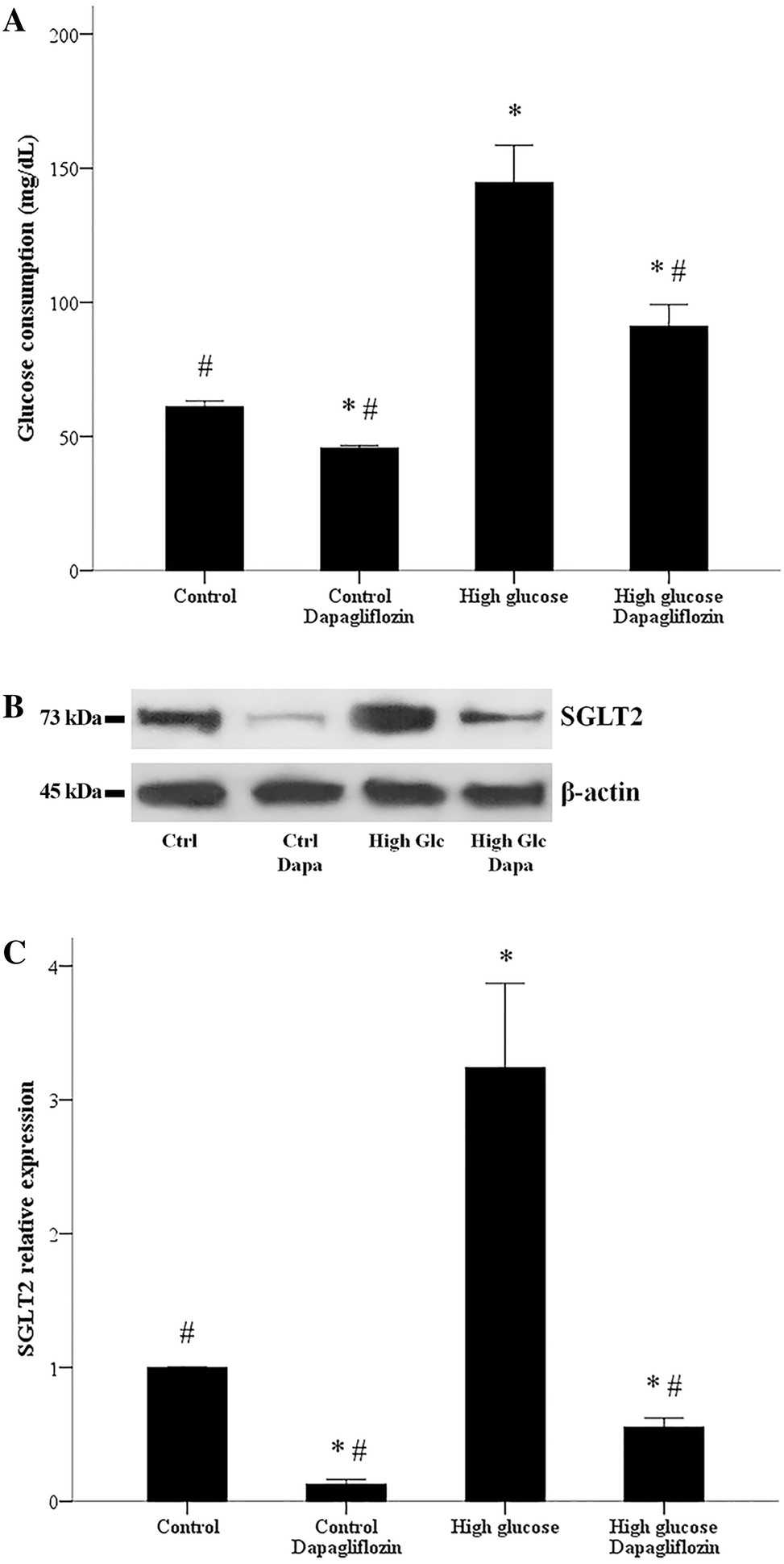 Fig. 1. Glucose influx and SGLT2 expression in RPTECs, and the effect of dapagliflozin. Compared to normal glucose, under high glucose conditions, the consumption of glucose by RPTECs increases (Eleftheriadis T, Pissas G, et al., 2020).
Fig. 1. Glucose influx and SGLT2 expression in RPTECs, and the effect of dapagliflozin. Compared to normal glucose, under high glucose conditions, the consumption of glucose by RPTECs increases (Eleftheriadis T, Pissas G, et al., 2020).
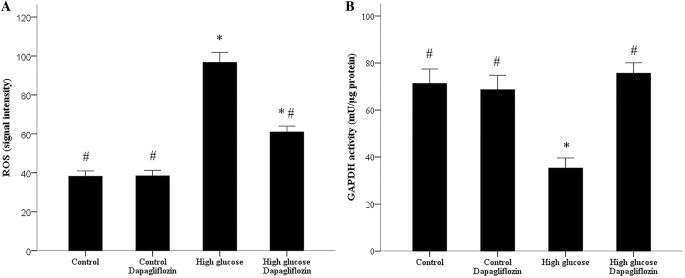 Fig. 2. ROS production and GAPDH activity in RPTECs and the effect of dapagliflozin (Eleftheriadis T, Pissas G, et al., 2020).
Fig. 2. ROS production and GAPDH activity in RPTECs and the effect of dapagliflozin (Eleftheriadis T, Pissas G, et al., 2020).
Organic Cation Transport Activity in Primary PTEC, RPTEC/TERT1, and PTL Cells
The renal proximal tubule is critical for drug transport and toxicity, relying on polarized transporter expression. Current animal models poorly predict human nephrotoxicity due to species differences, necessitating human in vitro models that replicate transporter functionality. Meijer et al. evaluates organic cation/anion transporter activity in three human proximal tubular models: iPSC-derived proximal tubular-like cells (PTL), human proximal tubular epithelial cells (PTEC), and human renal proximal tubular epithelial cells (RPTEC/TERT1), aiming to assess their utility for drug disposition and toxicity studies.
The functionality of organic cation transporters was assessed in transwell-cultured cells using fluorescent substrate ASP, with/without inhibitor quinidine. Basolateral ASP application induced time-dependent intracellular accumulation in all models (PTEC, RPTEC/TERT1, PTL), with basolateral uptake consistently exceeding apical uptake (Fig. 3a). Primary PTEC showed 1.4–2.2-fold higher intracellular ASP levels and 1.9–2.6-fold faster uptake rates compared to RPTEC/TERT1 and PTL within 10 minutes (Fig. 3a). Quinidine co-incubation reduced intracellular ASP levels to 59–82% across models (Fig. 3b). Apical efflux assessment revealed basolateral-to-apical ASP transfer inhibited by quinidine (apical ASP reduced to 37–65%; Fig. 3c). Apical-to-basolateral transfer was minimal, confirming basolateral-to-apical dominance.
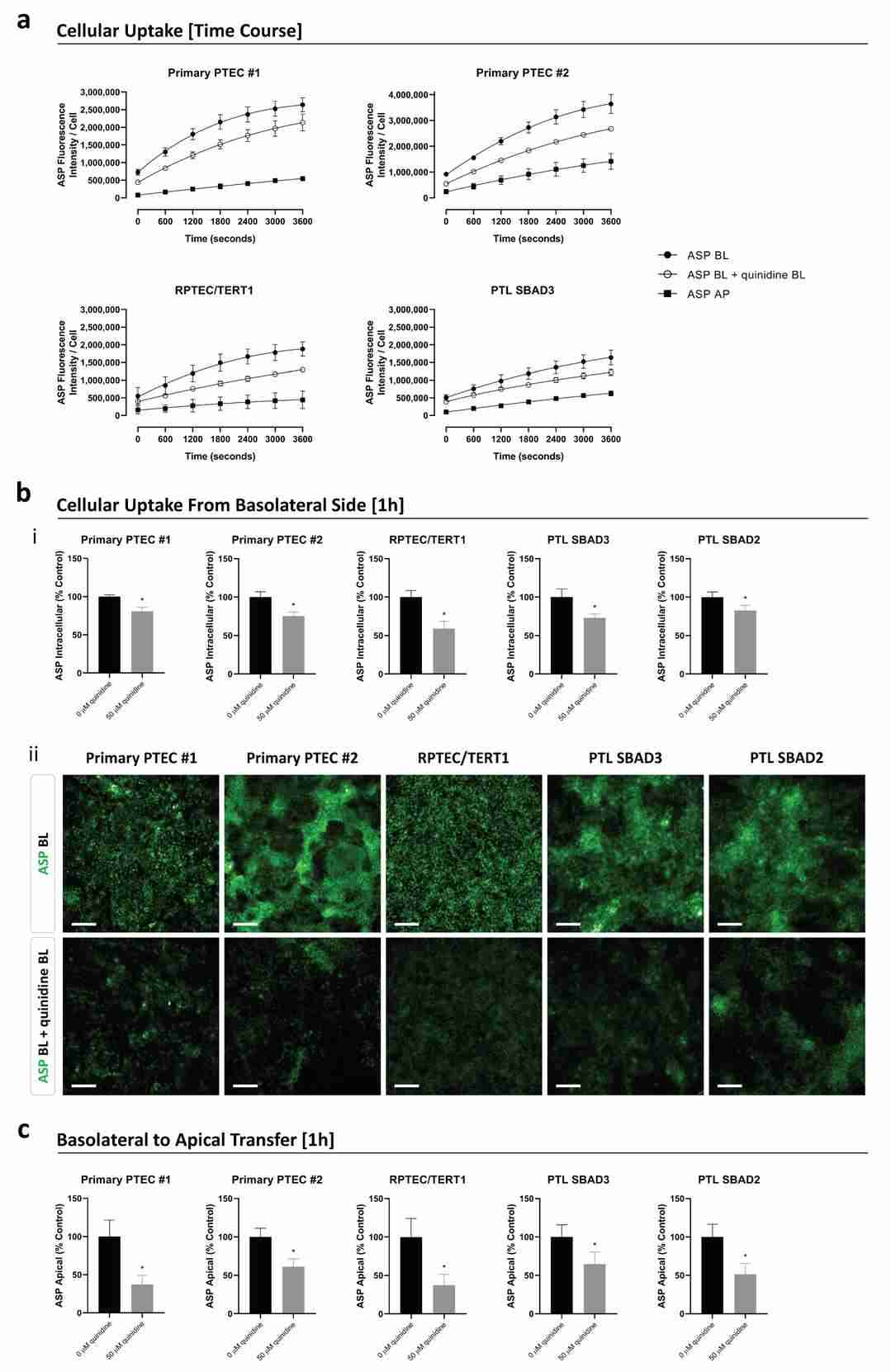 Fig. 3. The uptake and transfer of the fluorescent substrate 4-(4 (dimethylamino)styryl)-N-methylpyridinium iodide (ASP) in primary PTEC, RPTEC/TERT1, and PTL cells cultured on transwells (Meijer T, da Costa Pereira D, et al., 2024).
Fig. 3. The uptake and transfer of the fluorescent substrate 4-(4 (dimethylamino)styryl)-N-methylpyridinium iodide (ASP) in primary PTEC, RPTEC/TERT1, and PTL cells cultured on transwells (Meijer T, da Costa Pereira D, et al., 2024).
Ask a Question
Write your own review