Human Hair Follicular Keratinocytes (HHFK)
Cat.No.: CSC-7776W
Species: Human
Source: Hair Follicle
Cell Type: Keratinocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Hair follicular keratinocytes are specialized epithelial cells found in hair follicles, which are small structures located in the dermis of the skin. The main function of hair follicular keratinocytes is to ensure the continuous growth and renewal of hair. During the anagen phase of the hair growth cycle, these keratinocytes actively proliferate and differentiate to form the hair shaft. They undergo a complex process called keratinization, during which they synthesize and arrange keratin proteins into long, parallel bundles that give hair its strength and flexibility. In addition, hair follicular keratinocytes also contribute to the production of sebum, an oily substance that moisturizes and protects the hair and scalp. Apart from their role in hair growth and maintenance, hair follicular keratinocytes also participate in immune responses and wound healing. They act as a defense against external pathogens and provide a physical defense mechanism by producing antimicrobial peptides. Moreover, these keratinocytes can respond to injury or infection by initiating a series of cellular processes to repair damaged tissue and promote healing. They can migrate to the site of injury, proliferate, and differentiate to form new epidermal cells, ultimately leading to the regeneration of the hair follicle. By understanding the functions and behaviors of these specialized cells, we are able to gain valuable insights into the biology of hair follicles and their dynamic interactions with the surrounding microenvironment.
Evaluation of Cd-Induced Cytotoxicity in Primary Human Keratinocytes
Cd-induced dermatotoxicity is an important field of research, but most studies have been performed using HaCaT cells but not primary keratinocytes. In this study, we provide the results describing the cytotoxic effects of Cd exposure on primary human epidermal keratinocytes obtained from different donors. The subtoxic concentration of cadmium chloride was determined via MTT assay, and transcriptomic analysis of the cells exposed to this concentration (25 µM) was performed. As in HaCaT cells, Cd exposure resulted in increased ROS levels, cell cycle arrest, and induction of apoptosis. In addition, we report that exposure to Cd affects zinc and copper homeostasis, induces metallothionein expression, and activates various signaling pathways, including Nrf2, NF-kB, TRAIL, and PI3K. Cd induces the secretion of various cytokines (IL-1, IL-6, IL-10, and PGE2) and upregulates the expression of several cytokeratins, such as KRT6B, KRT6C, KRT16, and KRT17. The results provide a better understanding of the mechanisms of cadmium-induced cytotoxicity and its effect on human epidermal skin cells.
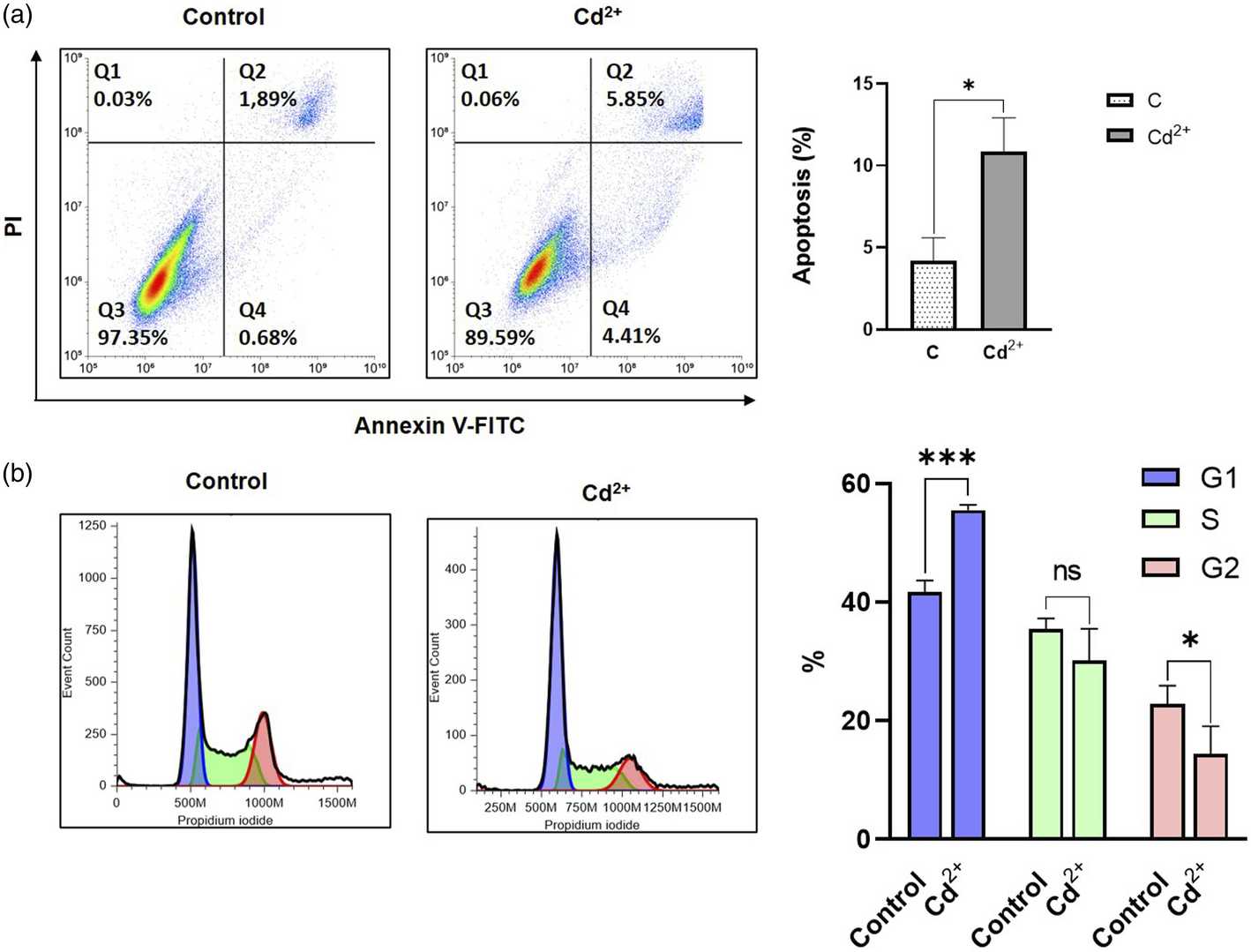 Fig. 1. Evaluation of apoptosis and cell cycle analysis after 24 h exposition with 25 µM of cadmium chloride (Romashin, Daniil, et al. 2024).
Fig. 1. Evaluation of apoptosis and cell cycle analysis after 24 h exposition with 25 µM of cadmium chloride (Romashin, Daniil, et al. 2024).
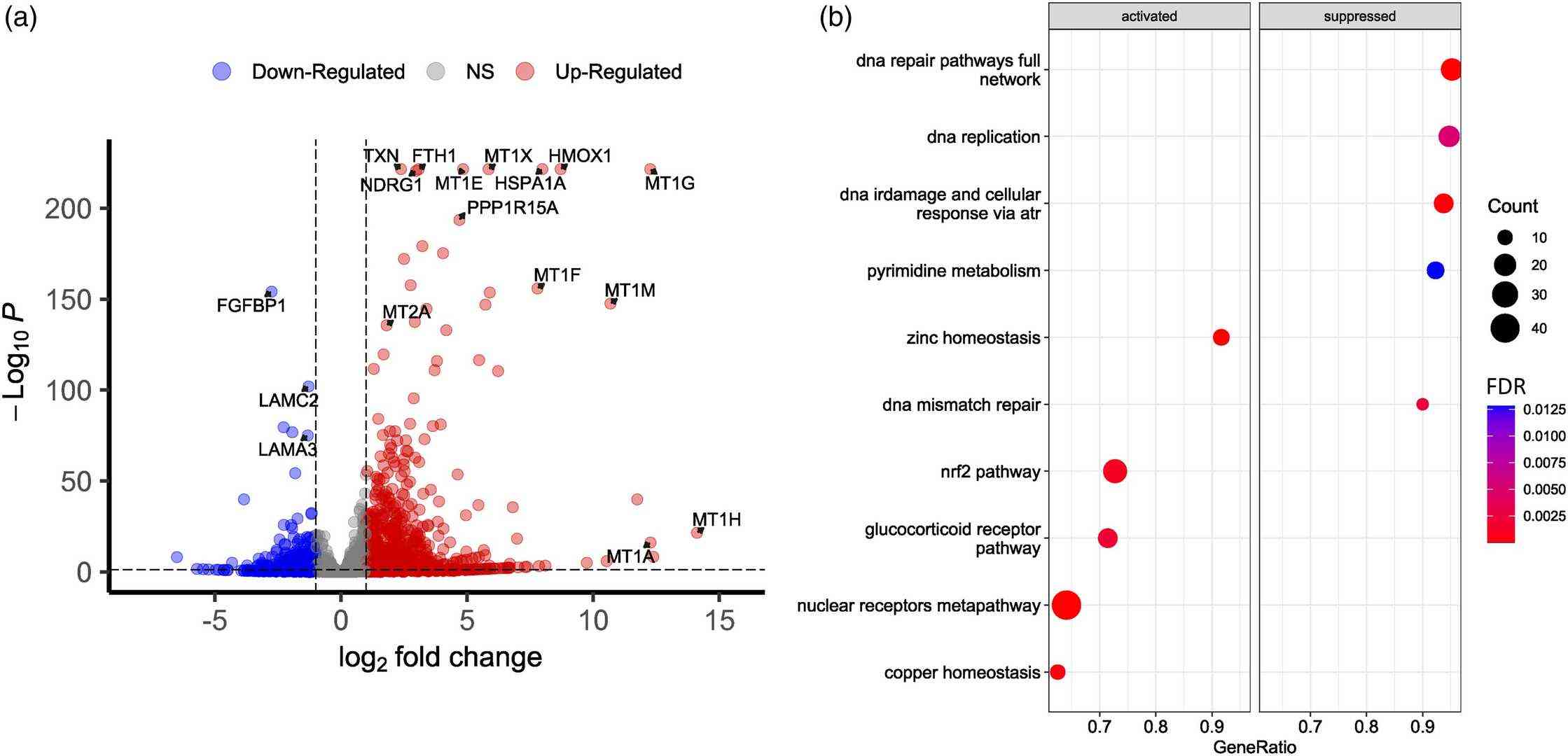 Fig. 2. Transcriptomic profiling of intact and Cd-treated normal human epidermal keratinocytes (Romashin, Daniil, et al. 2024).
Fig. 2. Transcriptomic profiling of intact and Cd-treated normal human epidermal keratinocytes (Romashin, Daniil, et al. 2024).
Glass, As Cell Culture Substrate, Enhances Proliferation and Migration of Human Keratinocytes
Human keratinocytes require relatively long propagation time which impedes their availability as autologous cell transplantation within a clinically reasonable timeframe. There is an unmet need for efficient xeno-free cell expansion approaches to propagate human keratinocytes as regenerative therapy.
Herein, we report a xeno-free and matrix-free culture substrate utilizing borosilicate-based glass in expanding monolayer of human keratinocytes. The efficacy of the glass substrate is compared with commercially available culture plastic and rat-tail collagen I coating. This study is an attempt to develop an EMA compliant expansion protocol which enables expeditious propagation of adult human keratinocytes in vitro to facilitate their scalability for bio-production.
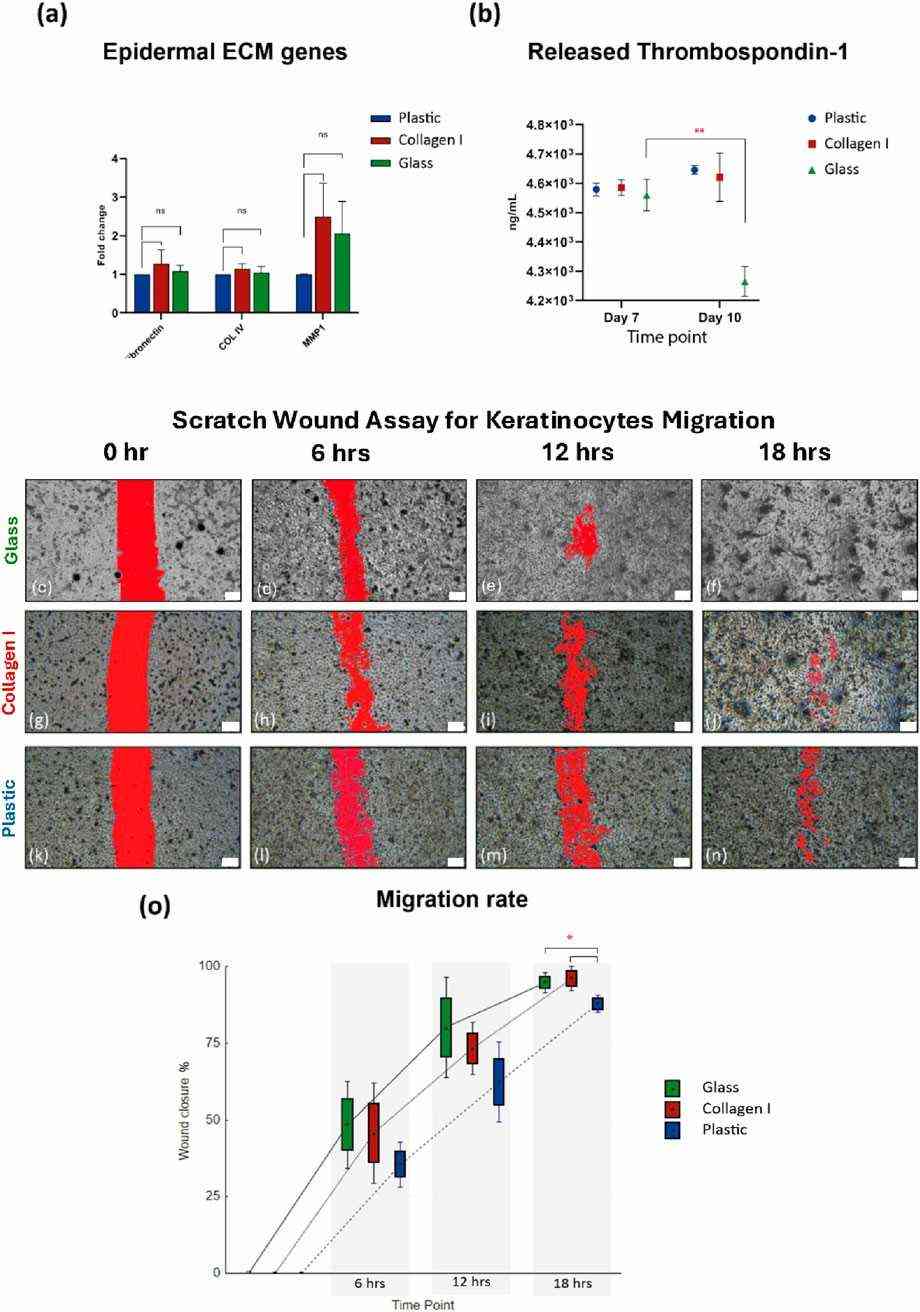 Fig. 3. (a) Gene expression of ECM markers. (b) Comparable amounts of thrombospondin-1 released by keratinocytes cultured on all three substrates. (c-o) Scratch wound assay for primary keratinocyte migration (Shahin, Hady, et al. 2025).
Fig. 3. (a) Gene expression of ECM markers. (b) Comparable amounts of thrombospondin-1 released by keratinocytes cultured on all three substrates. (c-o) Scratch wound assay for primary keratinocyte migration (Shahin, Hady, et al. 2025).
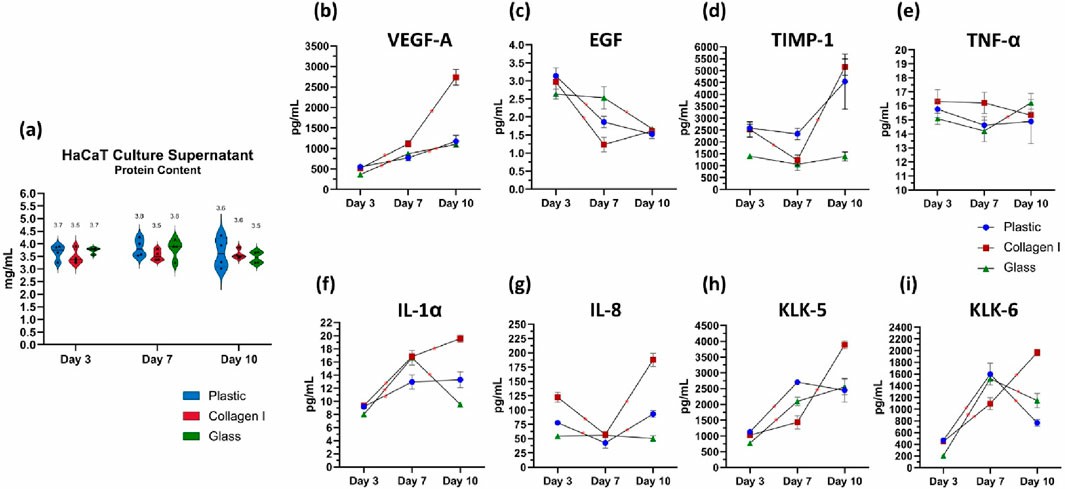 Fig. 4. Quantification of total protein content in the culture supernatant of keratinocytes (Shahin, Hady, et al. 2025).
Fig. 4. Quantification of total protein content in the culture supernatant of keratinocytes (Shahin, Hady, et al. 2025).
Ask a Question
Write your own review