Human Intestinal Smooth Muscle Cells (HISMC)
Cat.No.: CSC-7767W
Species: Human
Source: Intestine
Cell Type: Smooth Muscle Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Intestinal Smooth Muscle Cells (HISMCs) are primary cells isolated from the muscularis propria of the human small‑intestinal wall (most often the jejunum or ileum). HISMCs have an elongated, spindle‑shaped morphology, and express high levels of stress fibers (prominent actin filament bundles) that contain the classic smooth‑muscle markers α‑smooth muscle actin (α‑SMA), desmin, calponin and transgelin. They are contractile in vitro, and are commonly used in collagen‑gel contraction assays: when seeded in a collagen gel, HISMCs remodel the matrix and decrease the area of the gel within 48 h, which is a quantitative measure of contractile activity. HISMCs are responsive to multiple vasoactive agents, such as cholecystokinin, endothelin‑1 and TGF‑β3, with the latter significantly increasing gel contraction and up‑regulating the expression of contractile‑specific genes, making them a useful model for the hyper‑contractile environment that occurs in some congenital intestinal disorders. Their human origin and preservation of a smooth‑muscle phenotype make HISMCs an attractive model for the screening of pro‑kinetic or anti‑spasm drugs, and the mechanistic exploration of gut motility disorders and smooth‑muscle‑specific signaling pathways (Rho‑kinase, PKC, cAMP/PKA), as well as providing a cellular model for use in co‑culture systems with epithelial, neuronal, or immune cells to recreate the intestinal microenvironment (e.g., gut‑on‑chip).
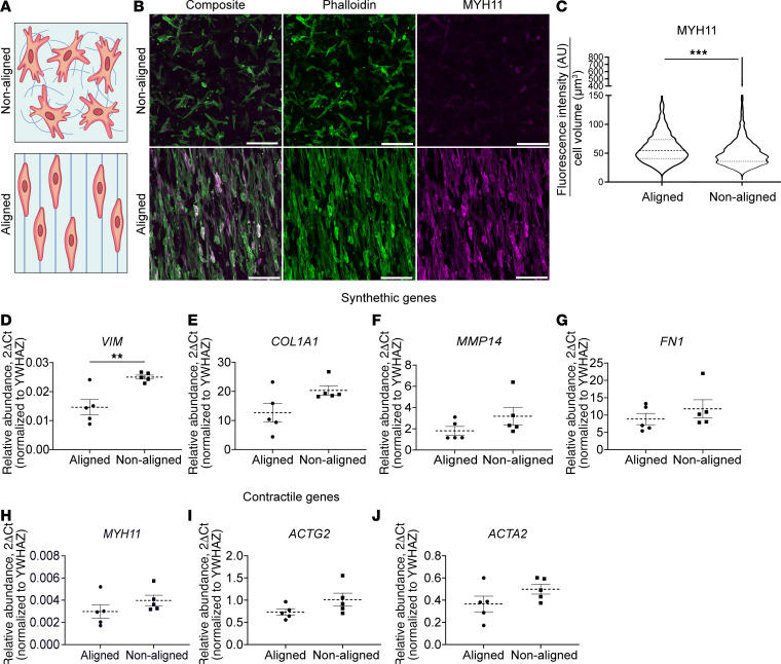
HISMCs Grown on Aligned Scaffolds have More Smooth Muscle Myosin Heavy Chain 11 (MYH11) Protein and Less VIM mRNA than HISMCs Grown on Nonaligned Scaffolds
Bowel smooth muscle experiences mechanical stress constantly during normal function and pathologic mechanical stressors in disease states. Wolfson et al. aim to test if pathological mechanical stress acutely alters gene expression in contractile bowel SMCs. A challenge is that SMCs cultured on hard plastic quickly switch from a "contractile" phenotype (expressing MYH11) to a "synthetic" phenotype that produces ECM, migrates, and proliferates. To study mechanical stress effects in a more contractile phenotype, they seeded HISMCs onto laminin-coated electrospun PCL scaffolds. One set had aligned fibers to promote elongated, spindle-shaped SMCs that are more contractile. Another set had nonaligned fibers (Fig. 1A, B). After 72 hours, cells were stained with MYH11 antibodies. HISMCs on aligned scaffolds had 14% more MYH11 protein than those on nonaligned scaffolds (aligned: 54.33 AU; nonaligned: 47.6 AU, median [IQR]) (Fig. 1B, C). Synthetic SMC marker Vimentin (VIM) mRNA was also less abundant in HISMCs on aligned scaffolds (Fig. 1D). However, mRNA levels for ECM-related (COL1A1, MMP14, FN1) and contractile apparatus genes (MYH11, ACTG2, ACTA2) were statistically equivalent between aligned and nonaligned scaffolds (Fig. 1E-J). Based on these findings, aligned scaffolds were used in further experiments.
HISMC are isolated from a healthy individual.
Human Intestinal Smooth Muscle Cells are guaranteed to further expand for 15 population doublings under the conditions provided by Creative Bioarray.
Ask a Question
Write your own review