HGF;Human Gingival Fibroblasts (HGF)
Cat.No.: CSC-7778W
Species: Human
Source: Gingiva; Periodontium
Morphology: Fibroblast
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human gingival fibroblasts (HGFs) are derived from human gingival tissue, which is the mucosal tissue covering the neck of the teeth and alveolar bone. The gingiva presents a pink appearance while maintaining a firm texture and elasticity. The primary cellular component of gingival tissue is fibroblasts which make up most of the periodontal connective tissue. HGFs demonstrate typical fibroblast characteristics with spindle-shaped or stellate forms which include extended projections and branching structures. These cells develop adherently in vitro and build up multiple cellular layers. Through successive culture generations cells expand in volume and density and evolve from short spindle-shaped forms into elongated cobblestone-like structures.
HGFs play a crucial role in the repair and maintenance of periodontal tissue. They can synthesize collagen, elastic fibers, and other extracellular matrix components, providing structural support to periodontal structures. Furthermore, HGFs participate in controlling inflammatory responses by releasing cytokines such as IL-6 and PGE2 which help to manage immune system reactions during periodontitis. Scientific research shows that HGFs possess regenerative capabilities for periodontal tissue which aids in bone and soft tissue healing. The simple cultivation process and functional diversity of HGFs make them widely used in scientific research. For example, these cells serve as disease models for periodontitis and cancer studies and provide seed cells for periodontal tissue engineering and bone regeneration research while also helping researchers assess how drugs like NBBA impact inflammatory elements such as IL-6 and PGE2 in periodontitis.
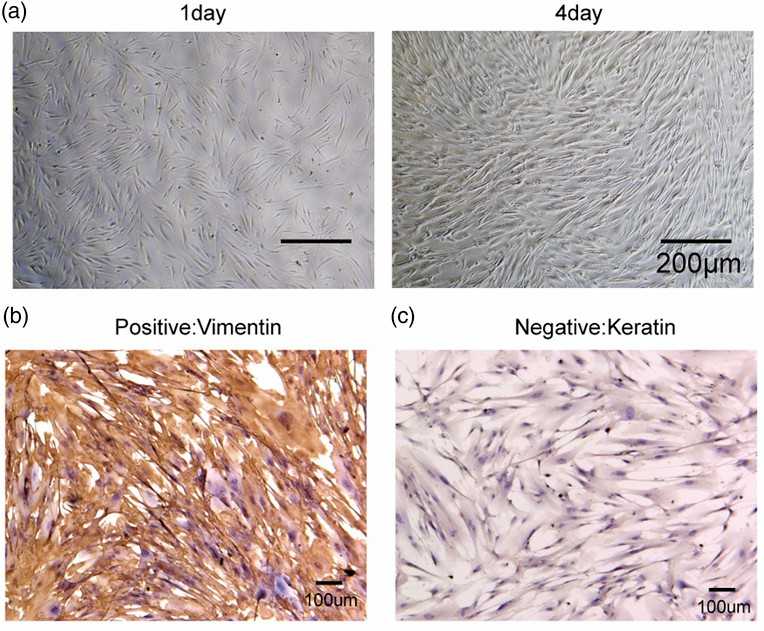 Fig. 1. Identification of human gingival fibroblasts (HGF-1). (A) Morphological observation of HGF-1 through a microscope (40×). (B and C) Detection of vimentin and cytokeratin in HGF-1 cells by IHC (Chen F C, Huang C M, et al., 2022).
Fig. 1. Identification of human gingival fibroblasts (HGF-1). (A) Morphological observation of HGF-1 through a microscope (40×). (B and C) Detection of vimentin and cytokeratin in HGF-1 cells by IHC (Chen F C, Huang C M, et al., 2022).
Long-Term Exposure to Nicotine or CSC Suppressed HGF Proliferation and Migration
Smokers face a high risk of periodontal disease because their periodontal tissues experience ongoing cigarette smoke exposure over extended periods. Tatusumi et al. examined how prolonged exposure to nicotine or cigarette smoke condensate (CSC) changes the cellular functions of human gingival fibroblasts (HGFs).
They first analyzed their effects on HGF proliferation. Cells were cultured short-term and long-term with specified nicotine or CSC doses, then counted and cultured for 24 hours. Figure 1 shows that long-term exposure significantly reduced HGF proliferation in a dose-dependent manner, while short-term exposure showed a non-significant trend of reduction. Cell migration was assessed using a wound healing assay. HGFs were counted after short-term or long-term culture with nicotine or CSC. Cell-free spaces were created following manufacturer instructions, and migration was observed for 24 hours. Long-term exposure significantly inhibited migration in a dose-dependent manner (Fig. 2), with no significant change in the short-term.
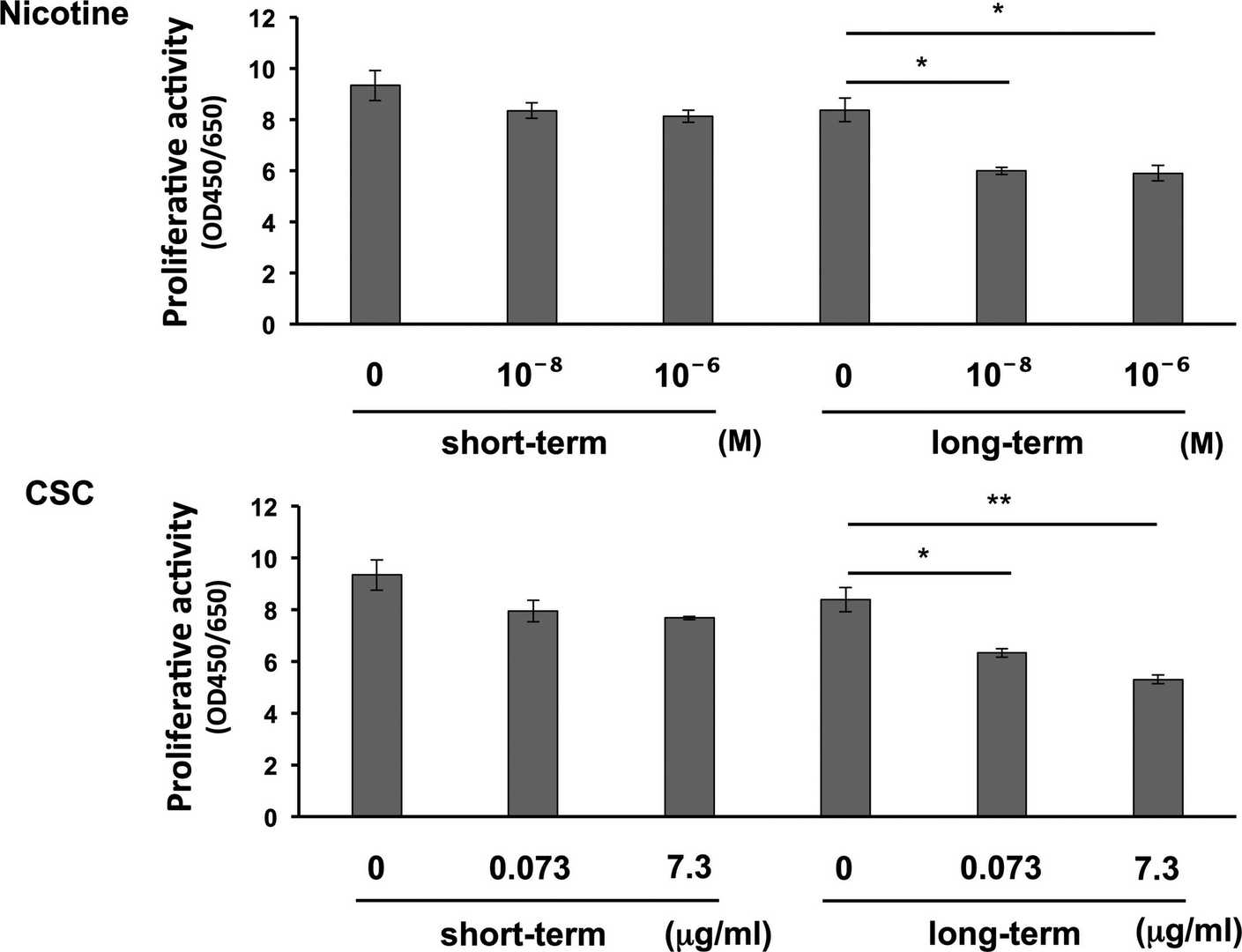 Fig. 1. Effects of long-term exposure to nicotine or cigarette smoke condensate (CSC) on human gingival fibroblast (HGF) proliferation (Tatusumi M, Yanagita M, et al., 2021).
Fig. 1. Effects of long-term exposure to nicotine or cigarette smoke condensate (CSC) on human gingival fibroblast (HGF) proliferation (Tatusumi M, Yanagita M, et al., 2021).
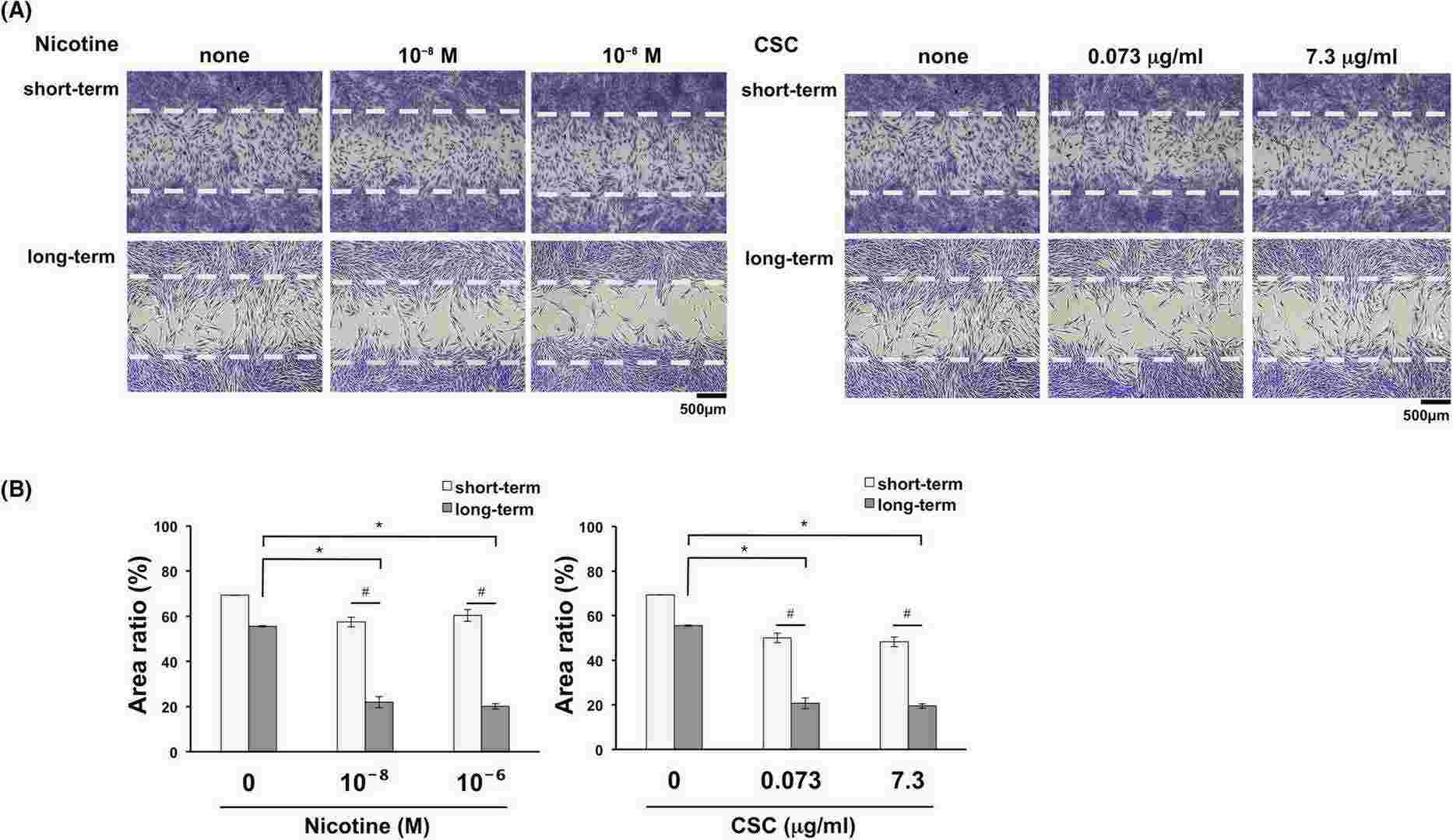 Fig. 2. Effects of long-term exposure to nicotine or cigarette smoke condensate (CSC) on cell migration ability (Tatusumi M, Yanagita M, et al., 2021).
Fig. 2. Effects of long-term exposure to nicotine or cigarette smoke condensate (CSC) on cell migration ability (Tatusumi M, Yanagita M, et al., 2021).
Substrate Stiffness Regulates Proinflammatory Responses in hGFs
Gum thickness affects the outcome of dental therapies, where thicker gingiva shows resistance to recession and periodontitis. Yet, the biological basis of gingival structure remains unclear. Given that ECM stiffness influences inflammatory conditions in fibroblasts, Tiskratok et al. investigates how substrate stiffness affects proinflammatory responses in human gingival fibroblasts (hGFs).
Two populations of human gingival fibroblasts (hGFs), labeled hGF-M2 and hGF-F1, were cultured on polydimethylsiloxane (PDMS) substrates with different stiffness levels (Young's modulus: 4.4, 17.0, and 26.2 kPa, see Fig. 3A). Coating with 0.1wt% collagen allowed hGF attachment. After 12 hours, hGF-M2 cells adhered equally across substrates (Fig. 3B), with similar nuclear and adherent cell numbers regardless of stiffness (Fig. 3B and C). Proinflammatory gene expression (PTGS2, IL1B) was higher on PDMS than polystyrene and decreased with increased stiffness, with the highest expression on soft PDMS (Fig. 3D). PGE2 levels on PDMS were also higher compared to polystyrene, decreasing as substrate stiffness increased, peaking on soft PDMS (Fig. 3E). With LPS co-incubation for 12 hours, hGF-M2 cells reached confluence on all substrates, similar to cultures without LPS on polystyrene (Fig. 3F). LPS markedly increased PTGS2, IL1B, and IL6 expression, particularly on PDMS substrates, with the highest levels on soft PDMS (Fig. 1G). PGE2 levels were also significantly higher on PDMS than polystyrene, with soft PDMS showing over 50% increase compared to other PDMS (Fig. 3H). In hGF-F1 cells, soft PDMS further amplified LPS-induced IL1B and PGE2 expression. Increased PDMS stiffness decreased LPS-induced proinflammatory response in hGF-F1 cells, reaching levels similar to polystyrene.
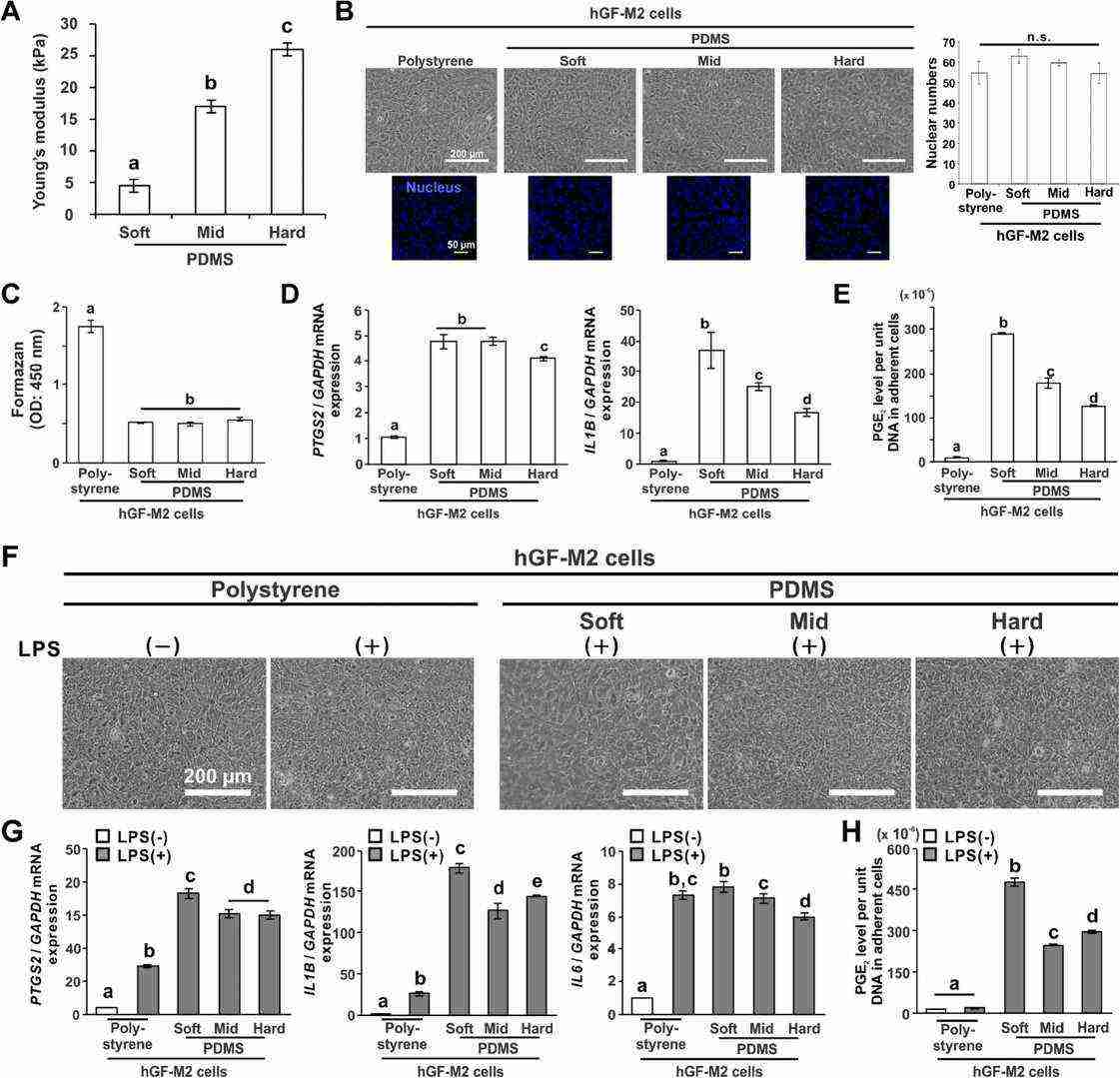 Fig. 3. Effects of substrate stiffness on the proinflammatory responses of hGFs (Tiskratok W, Yamada M, et al., 2023).
Fig. 3. Effects of substrate stiffness on the proinflammatory responses of hGFs (Tiskratok W, Yamada M, et al., 2023).
Ask a Question
Write your own review

