Human Peridontal Ligament Fibroblasts (HPLF)
Cat.No.: CSC-7738W
Species: Human
Source: Periodontal Ligament; Periodontium
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Periodontal Ligament Fibroblasts (HPDLFs) are the principal cellular constituents of the periodontal ligament (PDL), a specialized connective tissue that anchors the tooth root to the alveolar bone. These cells are not mere passive structural components but are highly dynamic and multifunctional. They originate from the dental follicle during tooth development and are uniquely positioned at the interface between two mineralized tissues-cementum and bone. Their primary role is to synthesize, maintain, and remodel the complex extracellular matrix (ECM) of the PDL, which is rich in collagens (notably type I and III), fibronectin, and proteoglycans. This ECM provides the unique biomechanical properties-a combination of toughness and shock absorption-essential for tooth function. Crucially, HPDLFs possess intrinsic osteogenic and cementogenic potential, playing a central role in the continuous remodeling of the periodontal attachment apparatus and in the healing responses following injury or disease.
The primary advantages of HPDLFs in research stem from their unique biological position and functional plasticity, making them indispensable for oral regenerative medicine and biomechanics.
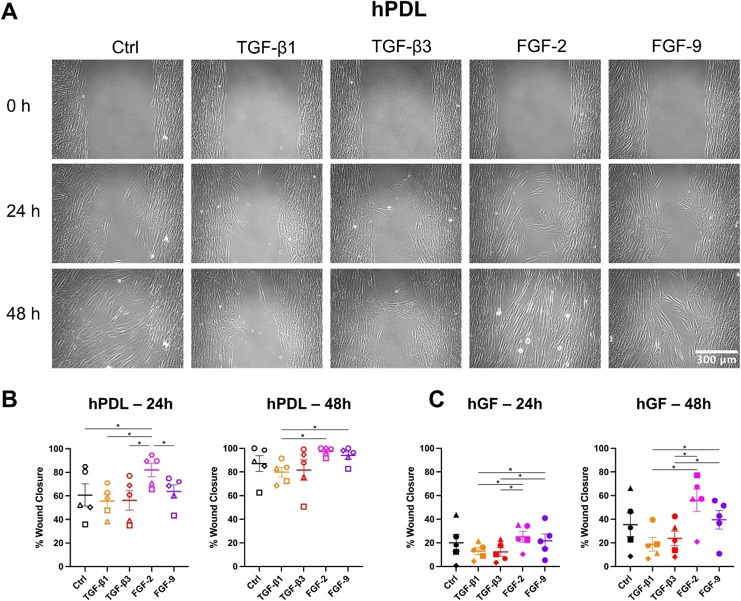
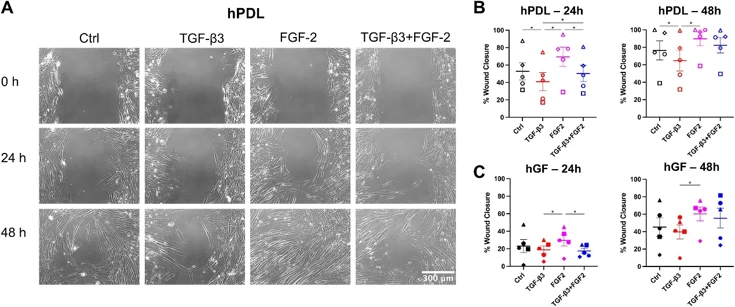
FGF-2+TGF-β3 Regulate Human Gingival and Periodontal Ligament Fibroblast Wound Healing Phenotype
Growth factor therapies for periodontal regeneration have gained traction, but quantification of their effects on multiple different fibroblast populations that are required for repair has been poorly investigated.
In this study, we examined the effects of TGF-β1, TGF-β3, FGF-2, and FGF-9 on human gingival fibroblasts (hGF) and human periodontal ligament cells (hPDL), as well as the combined effects of TGF-β3 and FGF-2. We show that FGF-2 enhances cell migration while TGF-β1 and TGF-β3 promotes matrix production, and TGF-β1 promotes fibroblast to myofibroblast transition. Interestingly, the combination of TGF-β3 and FGF-2, acting through both p-SMAD3 and p-ERK pathways, mitigates the inhibitory effects of TGF-β3 on migration in hPDL cells, suggesting synergistic and complimentary effects of FGF-2 and TGF-β3. Additionally, fibronectin production in hGF increased when treated with the combined TGF-β3+FGF-2 compared to FGF-2 alone, indicating that the effects of TGF-β3 in promoting extracellular matrix production are still active in the combined treatment condition. Finally, our study highlights that FGF-9 did not influence migration, α-SMA expression, or extracellular matrix production in either cell type, emphasizing the unique roles of specific growth factors in cellular responses.
Ask a Question
Write your own review