Canine Mammary Epithelial Cells
Cat.No.: CSC-C9119J
Species: Dog
Source: Breast
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Canine mammary epithelial cells originate from the mammary tissues of dogs. The mammary gland contains epithelial cells which create the ductal system and alveolar structures that produce and secrete milk. These cells are essential for synthesizing key milk components, including proteins such as casein and lactalbumin, as well as lactose, fatty acids, and antimicrobial compounds like lactoferrin. Notably, mammary epithelial cells possess remarkable differentiation potential, enabling them to adapt their shape and function in response to environmental changes within the body. Mammary cells experience substantial differentiation during pregnancy and lactation to enable milk production and discharge. Additionally, these cells demonstrate regenerative abilities which allow them to mend tissue damage by stem cell proliferation and differentiation.
Scientific studies use canine mammary cancer models to investigate breast cancer genesis and tumor environment interactions as well as treatment methods. The study of canine mammary cancer reveals shared cancer stem cell and embryonic stem cell-like properties with human breast cancer which offers essential information about breast cancer invasiveness. The exosomes produced by mammary epithelial cells influence breast cancer development and progression by controlling tumor microenvironment conditions and transferring miRNA molecules that affect tumor progression.
Malignant Canine Mammary Epithelial Cells Shed Exosomes Containing Differentially Expressed microRNA
Breast (mammary) cancers in human (BC) and canine (CMT) patients share clinical, pathological, and molecular similarities that suggest dogs may be a useful translational model. Many cancers, including BC, shed exosomes that contain microRNAs (miRs) into the microenvironment and circulation, and these may represent biomarkers of metastasis and tumor phenotype.
Fish et al. employed in vitro culture of normal and malignant canine mammary epithelial cells to isolate exosomes via ultracentrifugation. These exosomes were examined using electron microscopy, light scattering, and protein detection methods. The RNA bioanalyzer profiles showed a typical exosomal pattern, favoring small RNAs (~?20–200 nucleotides) with minimal rRNA (Fig. 1a). A total of 338 unique miRs were identified in the cell-free RNA from CMEC and CMT samples. Principal component analysis divided the eight samples into two distinct CMEC and CMT clusters, although two CMT samples were outliers (Fig. 1b). Volcano plot analysis demonstrated that many miRs were significantly over- or under-expressed in CMT exosomes compared to CMEC (Fig. 1c). With criteria of p?
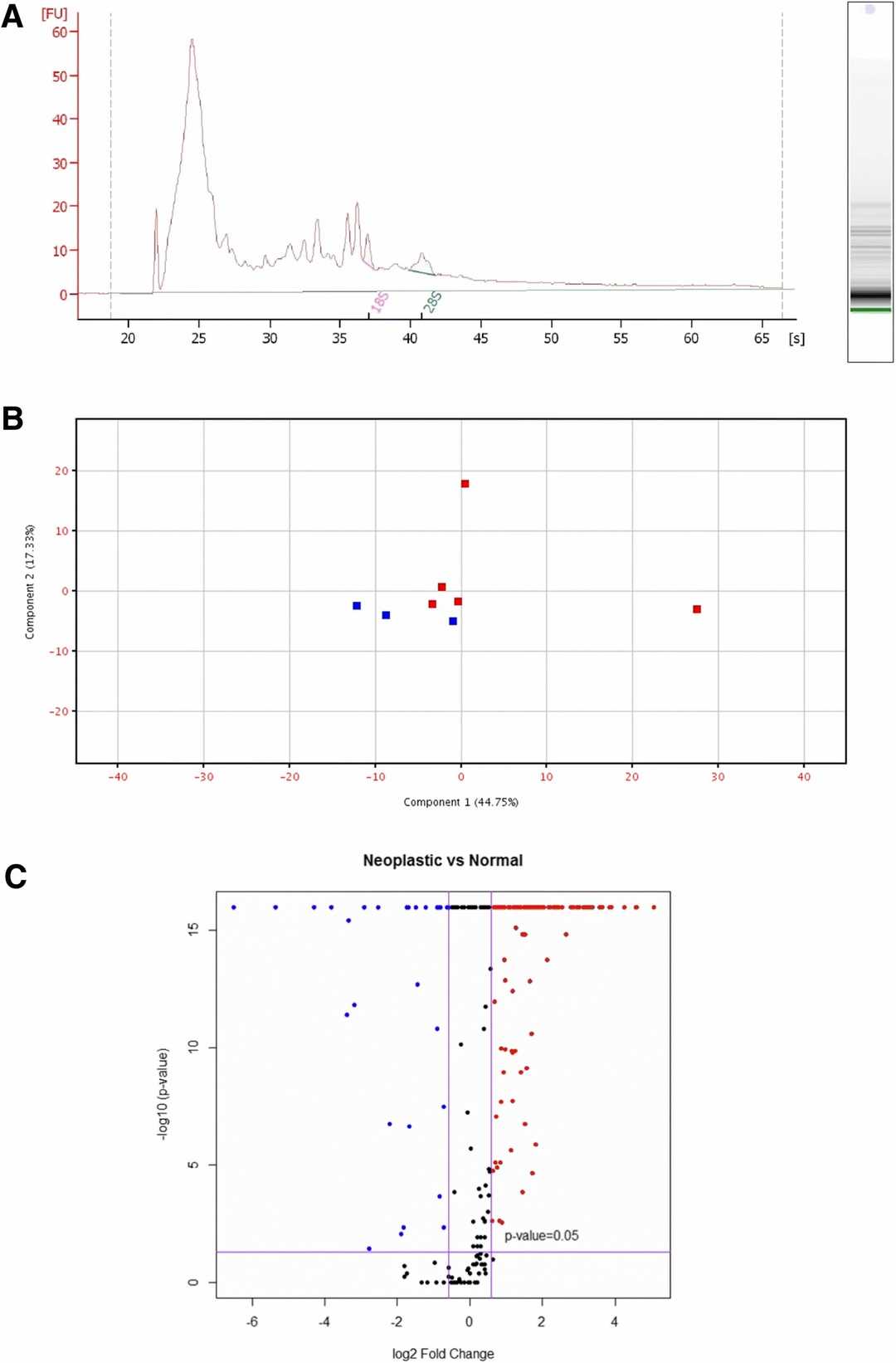 Fig. 1. RNAseq profiling exosomal RNA (Fish E J, Irizarry K J, et al., 2018).
Fig. 1. RNAseq profiling exosomal RNA (Fish E J, Irizarry K J, et al., 2018).
Targeting Canine Mammary Neoplastic Epithelial Cells with A Reengineered Anthrax Toxin
Mammary tumors, common in female dogs, resemble human breast cancer. da Fonseca et al. explored a reengineered anthrax toxin to treat these tumors without harming healthy cells. Given the role of metalloproteinases and urokinase in cancer metastasis, the toxin was modified to target these enzymes.
They employed five canine neoplastic epithelial cell lines and one normal cell line, treated with varying concentrations of the toxin. Cell cycle analysis, conducted via propidium iodide staining and flow cytometry, showed that the normal canine mammary epithelial cells (LOEC-NMG) and the LOEC-MCA1 mixed adenocarcinoma cell line had the lowest S phase proportions (5% and 5.12%). This suggests that less proliferative cells resist treatment better and are less affected by anthrax toxin. In their established cell lines, around 12% of adenoma (LOEC-MAd), complex adenocarcinoma (LOEC-MCA2), and simple adenocarcinoma (LOEC-MCA3) cells were in S phase. The adenocarcinoma cell line CF41.mg was the most proliferative, with 15.80% in S phase and 1.05 DNA index (Fig. 2). The G1 phase predominated across cell lines, with LOEC-MCA2 at 83.35% and LOEC-NMG at 89.4%. Cells had a DNA index between 1.00 and 1.05, denoting them as diploids, indicating low proliferation and favorable treatment response.
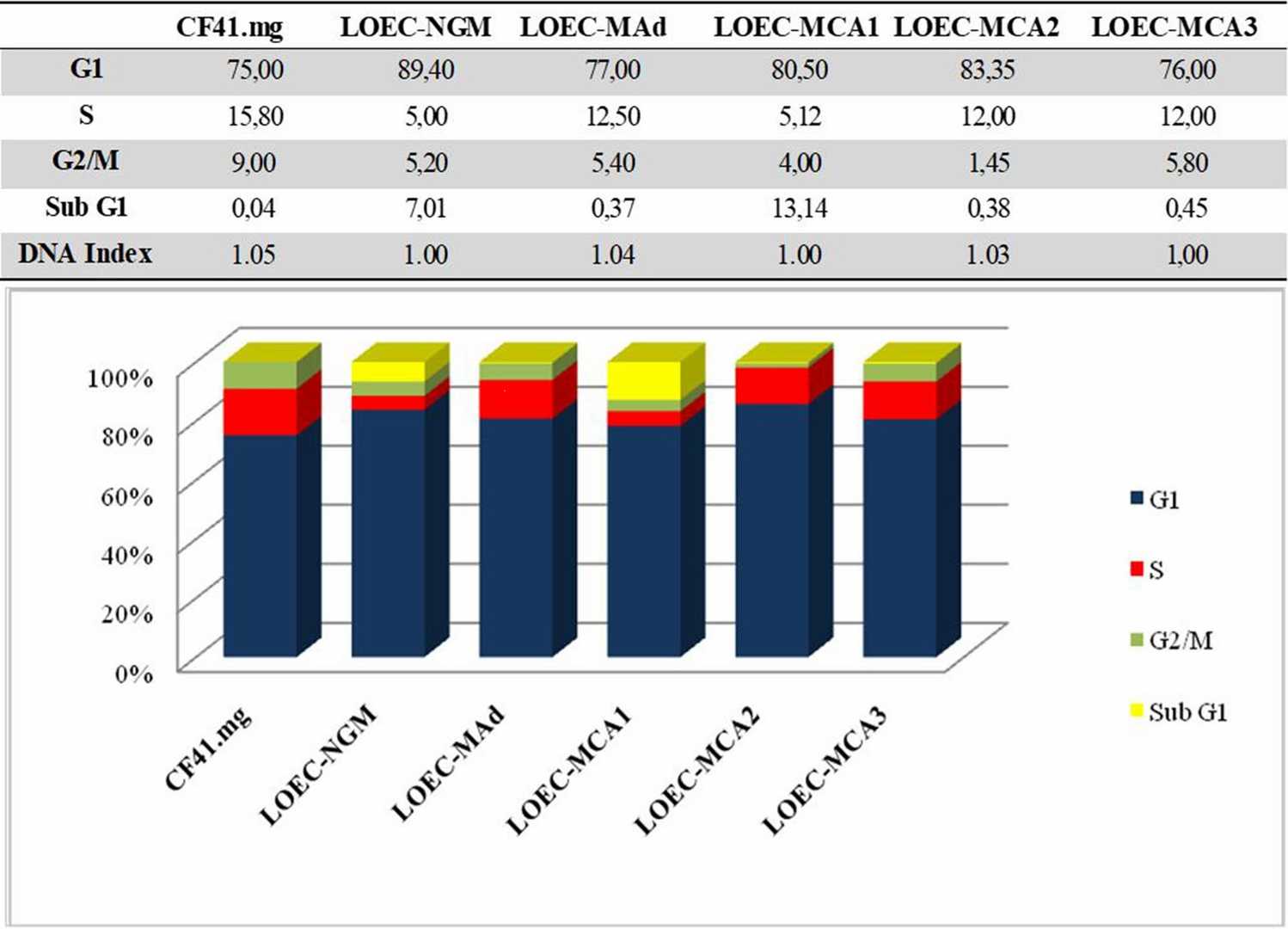 Fig. 2. Cell cycle analysis of canine mammary cell lines (da Fonseca I I M, Nagamine M K, et al., 2024).
Fig. 2. Cell cycle analysis of canine mammary cell lines (da Fonseca I I M, Nagamine M K, et al., 2024).
Ask a Question
Write your own review