Canine Cardiac Fibroblasts
Cat.No.: CSC-C4802L
Species: Dog
Source: Heart
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
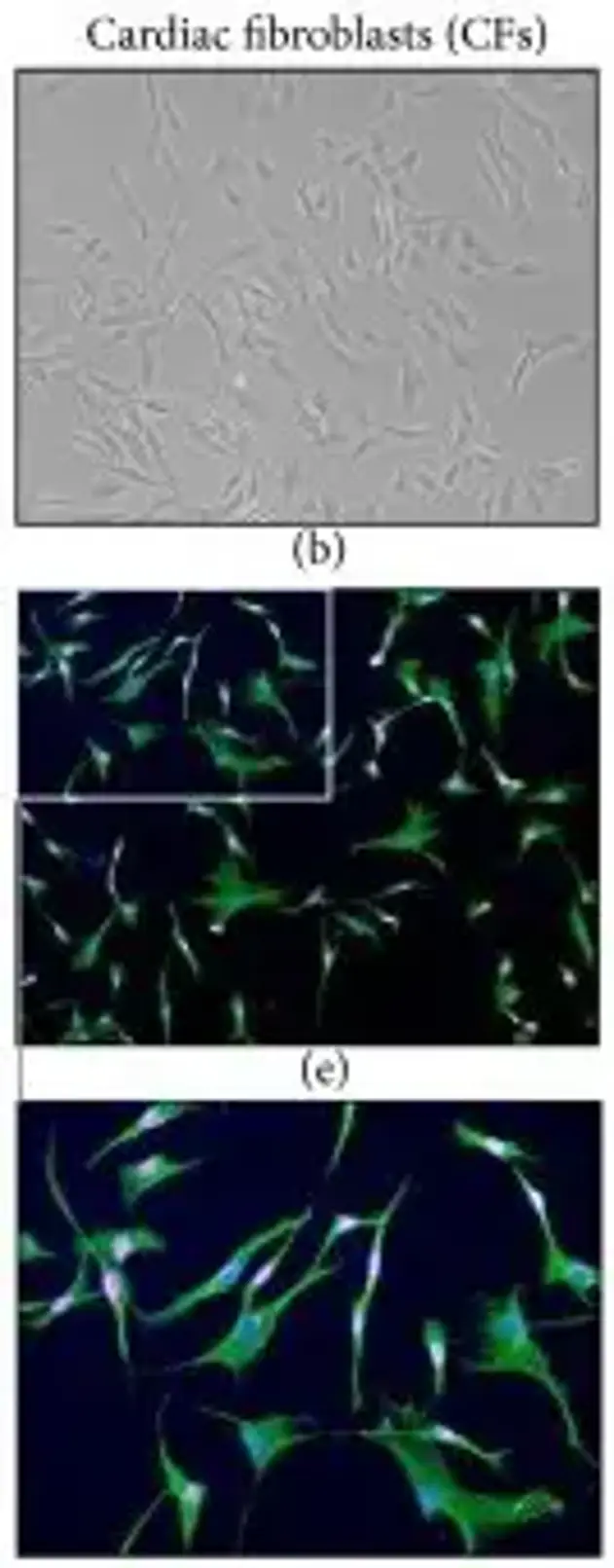
Canine Cardiac Fibroblasts are primary cells that are isolated from dog heart. They are a major non-myocyte cell type present in the heart. Cardiac fibroblasts have been shown to play a central role in maintaining cardiac structure, which contributes to myocardial stiffness and mechano-signaling. Canine cardiac fibroblasts express the characteristic fibroblast morphology. As well as expressing markers that are typical for fibroblasts such as vimentin, discoid in domain receptor 2 (DDR2), collagens (types I and III) and fibronectin. In addition, upon stimulation with profibrotic factors (such as transforming growth factor-β (TGF-β) or mechanical stretch) canine cardiac fibroblasts can also be induced to an activated myofibroblast phenotype (expressing increased α-smooth muscle actin (α-SMA) with an increased ECM deposition).
Canine cardiac fibroblasts have been used in research to study molecular pathways and mechanisms of cardiac fibrosis, cardiac hypertrophy, cardiac inflammation and arrhythmogenesis. The canine heart is very similar to the human heart with similar size, electrophysiology and pharmacological properties and thus canine cardiac fibroblasts are a very translational in vitro model. They have therefore been used to assess antifibrotic drugs, study cardiac toxicity, and develop preclinical drugs for treating cardiovascular diseases.
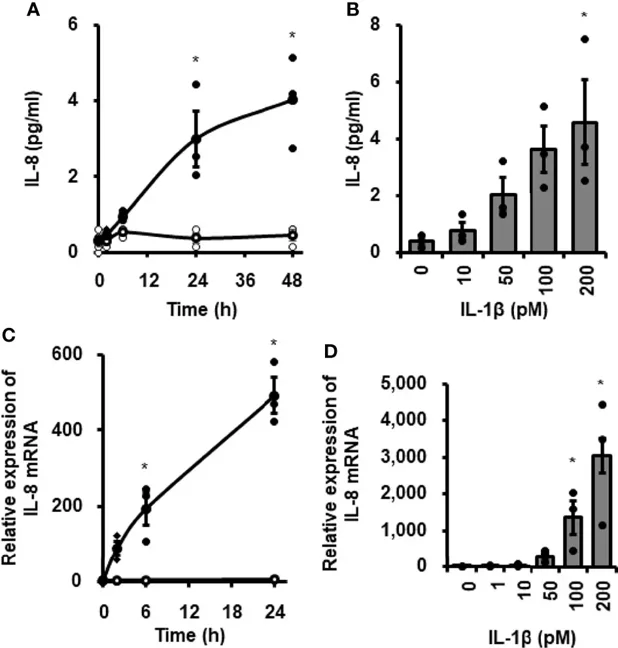
Inhibitory Effect of TPCA-1 on IL-1β-Mediated IL-8 mRNA Expression and Protein Release
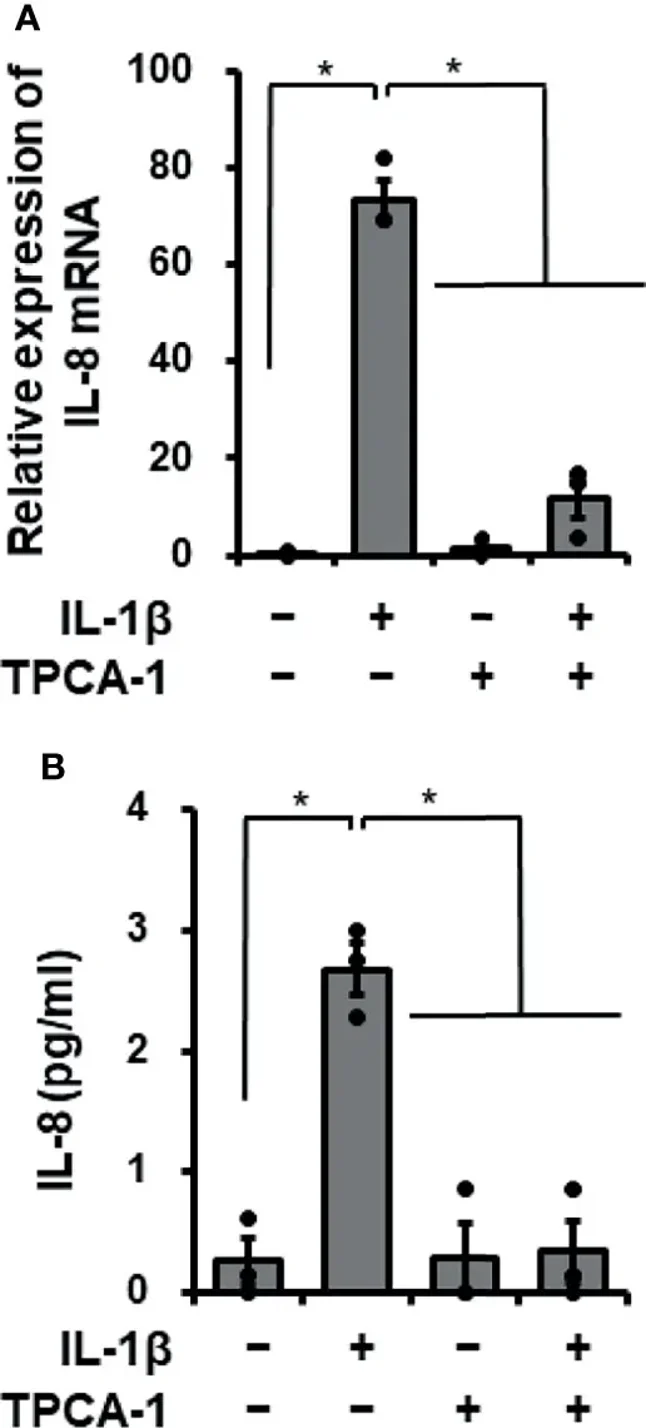
Cardiac fibroblasts play a role in heart disease inflammation. Mizuno et al. investigated how the proinflammatory cytokine IL-1β affects IL-8 expression, which contributes to innate immunity by recruiting neutrophils, in canine cardiac fibroblasts.
When canine cardiac fibroblasts were incubated with 100 pM IL-1β for 0-48 h, IL-8 concentration in the medium increased over time (Fig. 1A). In fibroblasts treated with 0-200 pM IL-1β for 24 h, IL-8 release increased with higher IL-1β concentrations (Fig. 1B). IL-1β also stimulated IL-8 mRNA expression in a time- and dose-dependent manner (Fig. 1C, D). These results suggest that IL-1β induces IL-8 expression and release in canine cardiac fibroblasts. Since IL-1β activates NF-κB to regulate mRNA expression, they used the NF-κB inhibitor TPCA-1 to examine its role in IL-1β-mediated IL-8 expression. Pretreating fibroblasts with 10 μM TPCA-1 for 1 h significantly reduced IL-1β's effects on IL-8 mRNA expression (Fig. 2A) and IL-8 release (Fig. 2B). This indicates that NF-κB activation is involved in IL-1β-induced IL-8 expression in these cells.
Ask a Question
Write your own review