GFP Expressing Human Dermal Fibroblasts-Adult (GFP-HDFCs-Ad)
Cat.No.: CSC-C5468W
Species: Human
Source: Dermis; Skin
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Dermal Fibroblasts (GFP-HDFs-) from Creative Bioarray, were isolated from the dermis of adult human skin and infected with lentiviral vectors that express green fluorescent protein (GFP). They are spindle-shaped or stellate cells, packed with long cytoplasm. HDFs respond to injury or inflammation by proliferating, moving and releasing components of the extracellular matrix, contributing to the healing and regeneration of wounds. Despite being unable to differentiate into other cell types, HDFs can be modified specifically to become induced pluripotent stem cells (iPSCs) capable of dividing into cell types.
Because they carry GFP, the cells turn green when in the dark, are easy to detect and follow across tissue. By monitoring the GFP fluorescence signal, scientists can monitor HDFs' proliferation, migration and differentiation, both in vitro and in vivo, and thus track these cells' culture-dependent movement and repair and regeneration in real time. Due to their sensitiveness to drugs and toxins, GFP-expressing HDFs cells are also used for drug screens and toxicological analysis. Tracking GFP fluorescence signal changes allows a fast measurement of how drugs or toxins impact HDFs, which is valuable for drug discovery and safety analyses.
Chemically Induced Transformation of Human Dermal Fibroblasts to Hair‐Inducing Dermal Papilla‐Like Cells
The Dermal Papilla (DP) at the base of the hair follicle (HF) is a specialized fibroblast that plays a crucial role in HF regeneration. However, the inductive capability of DP is limited during in vivo culture. Recent studies have shown that treating adult fibroblasts with acellular extracts from embryonic skin endows them with the ability to regenerate hair follicles. Moreover, epidermal FGF20 and PDGF‐A are involved in regulating DP formation, as well as Shh; TGF‐β2 and BMP7 secreted by β‐catenin-over-activating epidermal cells direct ectopic HF formation. Additionally, the administration of FGF2, BMP2, BIO, and suspension culture in vivo has shown to maintain the hair-inductive activity of DP cells. Therefore, Zhao et al. applied these candidate factors (Shh, PDGF, FGF20, TGF-β2, BMP7, FGF2, BMP2, BIO) and suspension cultures for the conversion of human dermal fibroblasts into DP-like cells.
Results indicate that the combination of FGF2, PDGF, and BIO for 6 days in adherent culture, followed by suspension culture for 24 hours, effectively induces transformation in both human fetal and adult fibroblasts (Fig. 1A). Post-treatment, fetal fibroblasts displayed a change in morphology (Fig. 1B). RT‐PCR, immunohistochemistry, and Western blot analyses confirmed upregulation of DP signature genes (Figure 1C, S3). Adult fibroblasts exhibited similar trends (Fig. 1D, E). Transformed fibroblasts acquired DP‐like characteristics. To evaluate the hair-inducing potential of DP‐like cells, a "patch assay" was conducted (Fig. 1F), with human dermal fibroblasts labeled with EGFP (EGFP-HDF) before implantation into nude mice alongside RFP-expressing neonatal mouse epidermal cells. At three weeks post-implantation, hair follicle (HF) structures formed in 65% of mice with fetal DP‐like cells and 70% with adult DP‐like cells (Fig. 1G). Both RFP+ epidermal and EGFP+ dermal cells were present in mice with DP‐like cell implants, unlike controls. Section assays revealed fetal/adult DP‐like cells integrated into DP and dermal sheath, contributing to new HFs (Fig. 1H). Furthermore, mCherry-labeled fibroblasts, in combination with K14‐H2B‐GFP mouse epidermal cells, confirmed that transformed cells integrated into regenerated HFs, indicating acquired hair follicle-inducing capabilities (Fig. 1H).
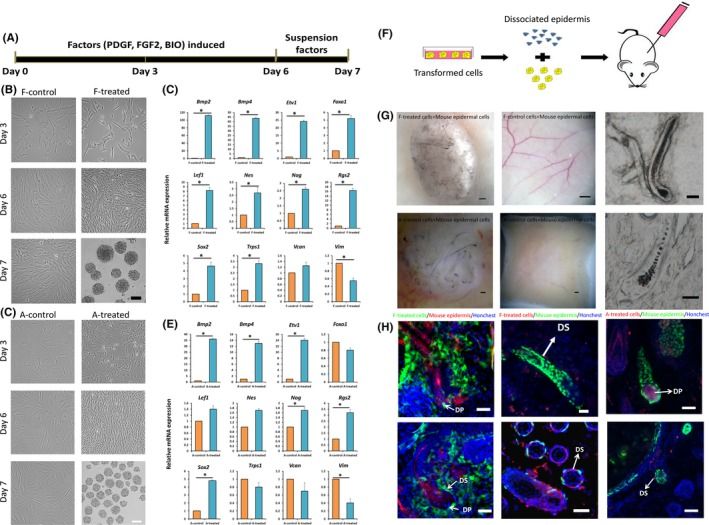 Fig. 1. Chemically induced transformation of human fibroblasts to hair‐inducing DP‐like cells (Zhao Q, Li N, et al., 2019).
Fig. 1. Chemically induced transformation of human fibroblasts to hair‐inducing DP‐like cells (Zhao Q, Li N, et al., 2019).
Re-expression of HDAC7 Delays the Occurrence of the Cell Cycle Arrest in Pre-senescent Dermal Fibroblasts
Senescent cells, initially considered to be in permanent growth arrest in vivo, are now linked to aging both normally and pathologically in vivo. Gene expression changes, influenced by epigenetic factors, including histone acetylation, involve a balance between histone acetyltransferases (HATs) and histone deacetylases (HDACs). HDAC activity generally diminishes with replicative senescence. Warnon's team investigates the role and expression of HDACs in the senescent phenotype of human dermal fibroblasts (HDFs).
SAHA, also known as Vorinostat, is a pan-HDACs inhibitor. Previous studies have shown that inhibiting HDACs using SAHA or siRNA accelerates senescence in dermal fibroblasts. To explore the effect of re-expressing HDAC2 or HDAC7 in pre-senescent cells, AG04431 HDFs and NHDFs (normal HDFs) were infected with lentivirus expressing either HDAC2 (pLV HDAC2), HDAC7 (pLV HDAC7), or EGFP (pLV EGFP) as a control. Western blot confirmed increased HDAC2 or HDAC7 protein levels in transduced cells (Fig. 2A and B, Figure 3A and B). Post-transduction, cells were passaged until reaching senescence. Analysis was conducted at passage 4 post-transduction (day 28 for pLV HDAC7, day 36 for controls). Cells transduced with pLV HDAC7 showed resumed proliferation and extended lifespan, evidenced by increased cell numbers (Fig. 2C, Fig. 3C) and higher Ki-67 positivity (Fig. 2D and E). There was also a decrease in SA-βgal positive cells (Fig. 2F, Fig. 3D). Morphologically, these cells resembled young, spindle-shaped cells. However, there was an unexpected increase in SASP factors IL-6, IL-8, MMP-1, and MMP-3 expression in pLV HDAC7 transduced cells (Fig. 2G). Re-expressing HDAC7 extends proliferation and reduces senescence-associated markers without lowering SASP factors.
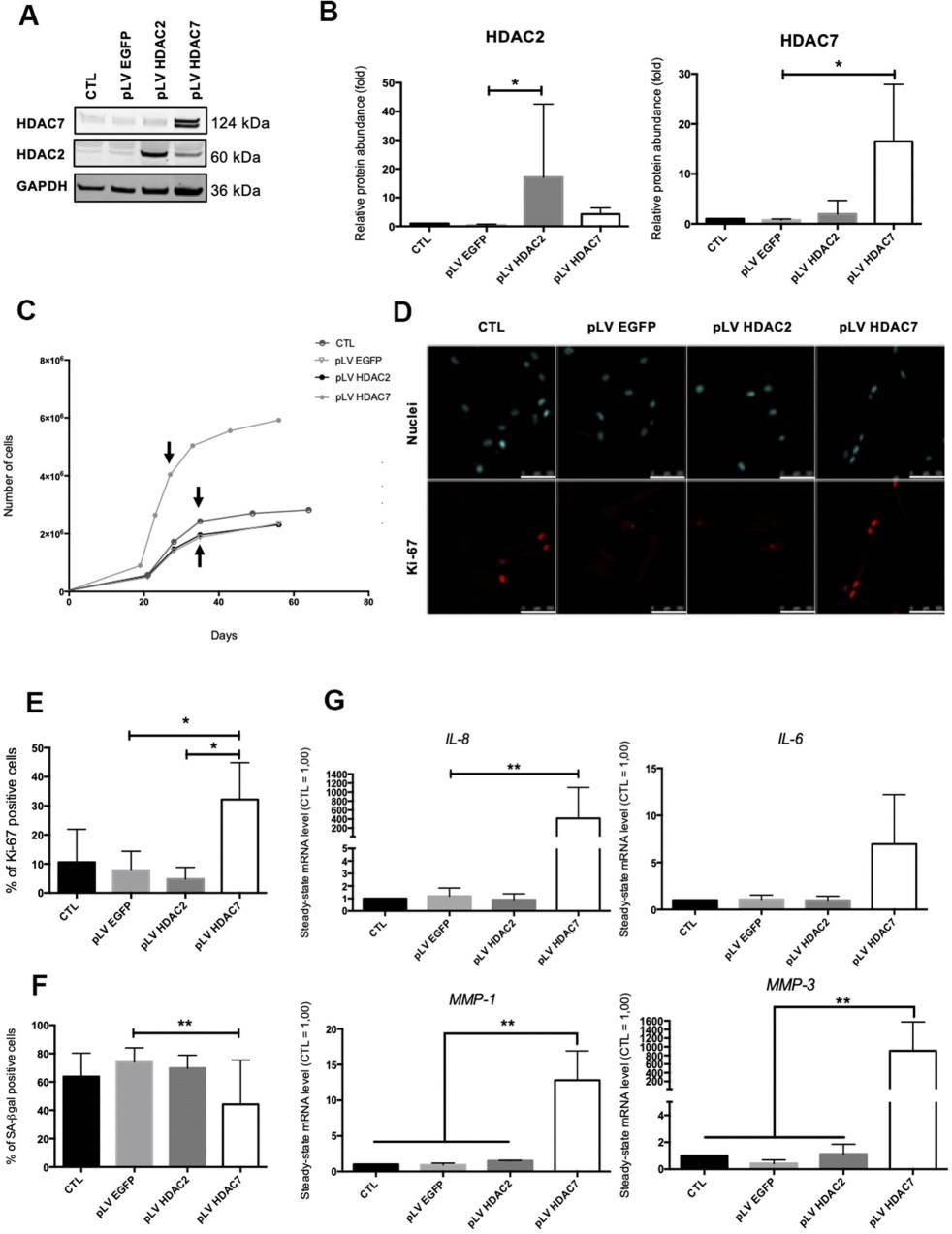 Fig. 2. HDAC7 but not HDAC2 re-expression allows to resume proliferation in pre-senescent cells (Warnon C, Bouhjar K, et al., 2021).
Fig. 2. HDAC7 but not HDAC2 re-expression allows to resume proliferation in pre-senescent cells (Warnon C, Bouhjar K, et al., 2021).
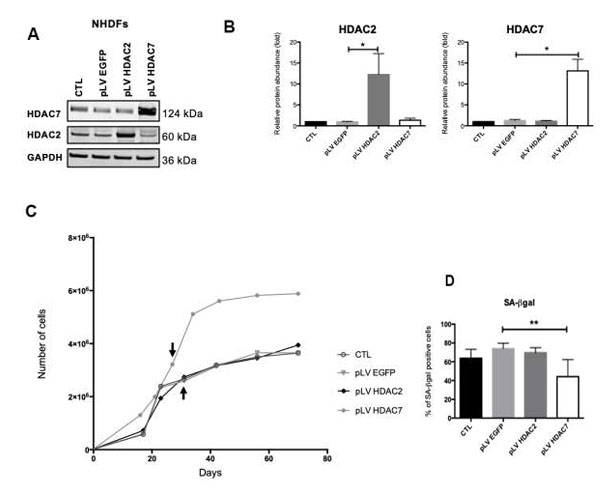 Fig. 3. HDAC7 but not HDAC2 re-expression allows to resume proliferation and to decrease the proportion of SA-βgal positive cells in pre-senescent cells. (Warnon C, Bouhjar K, et al., 2021).
Fig. 3. HDAC7 but not HDAC2 re-expression allows to resume proliferation and to decrease the proportion of SA-βgal positive cells in pre-senescent cells. (Warnon C, Bouhjar K, et al., 2021).
Ask a Question
Write your own review