Normal Human Dermal Fibroblasts-Neonatal
Cat.No.: CSC-C4121X
Species: Human
Source: Dermis; Skin
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Cell Features:
HDFn-XF are cryopreserved as primary cells. Cells are isolated from human foreskin and expanded in culture vessels once before being harvested for cryopreservation.
HDFn-XF are not exposed to phenol red or antimicrobials when cultured in FibroLife Xeno-Free Complete Medium.
HDFn-XF are not exposed to any animal*-derived material during isolation or when cultured in FibroLife Xeno-Free Complete Medium.
HDFn-XF are extensively tested for quality and optimal performance and xeno-free status of all materials used in the isolation, expansion and cryopreservation of each lot is documented.
Creative Bioarray guarantees performance and quality.
Normal Human Dermal Fibroblasts-Neonatal (NHDF-Neo) are primary human fibroblasts originally sourced from the dermis of newborn foreskin. Fibroblasts are the most abundant cell type in connective tissue of the skin. They have a spindle-like shape with two opposing ends, often referred to as bipolar, and they adhere well to standard culture plastic. Additionally, since these cells are taken from newborns rather than adults, they have been found to have increased proliferative abilities and are more resistant to becoming senescent in cell culture, which allows for extensive research and provides researchers with very consistent cell lines. As stated above, NHDFs contribute to the structural support of the skin. They produce and orderly secrete extracellular matrix proteins such as type I collagen, elastin and glycosaminoglycans. Furthermore, they aid in wound healing by migrating to wound sites and producing various growth factors and cytokines that modulate epidermal cells.
NHDF-Neo's can be used for many different applications due to their consistency. They are commonly used for dermatological research to study skin aging, UV damage, and tissue engineering when creating 3D skin models. They are also used as a baseline model to test drug and cosmetic toxicity on humans instead of using animal models.
Development of Fibroblast Differentiation Protocol Compatible with Clinical Grade Regulatory Standards
Chronic wounds, like leg ulcers in sickle cell disease, are hard to heal due to prolonged inflammation and lack effective treatments. Domingues et al. aims to develop novel cell- and tissue-based therapies using pluripotent stem cell-derived keratinocytes and fibroblasts to reconstruct dermo-epidermal substitutes with a plasma-based fibrin matrix for treating chronic wounds.
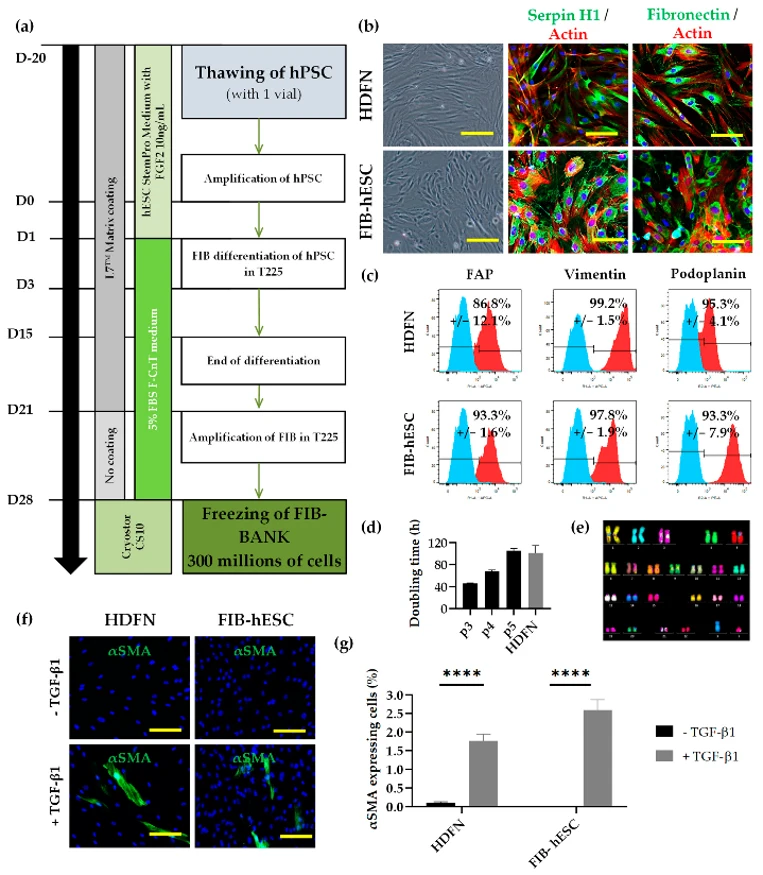
They developed a GMPc-compliant process to differentiate clinical hESCs into a homogeneous fibroblast population. The cells were cultured for 14 days, then mass-cultured to produce 300 million FIB-hESCs, which were frozen and later thawed for phenotype and functionality checks, enabling the production of around 15,000 cm² of dermal tissue. Microscopy analysis showed that the cells resembled normal neonatal human dermal fibroblasts (HDFN) in morphology (Fig. 1b). These cells expressed fibroblast markers serpin H1 and fibronectin. Flow cytometry revealed that over 95% of the cells were positive for mesenchymal markers CD73 and CD166, and over 85% were positive for FAP with over 95% positive for vimentin (Fig. 1c). Additionally, over 90% of the cells were podoplanin-positive (Fig. 1c). The doubling time of FIB-hESC was 47 hours at passage 3, faster than HDFN at passage 4 (101 hours) (Fig. 1d), but aligned with HDFN by passage 5. The cell karyotype remained normal post-differentiation (Fig. 1e). After TGF-β1 treatment for 4 days, 1.8% of HDFN and 2.6% of FIB-hESC were αSMA positive, indicating myofibroblast differentiation (Fig. 1f, g).
Ask a Question
Write your own review