Rat Schwann Cells
Cat.No.: CSC-C1795
Species: Rat
Cell Type: Schwann Cell; Glial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
RSC are isolated from neonatal rat sciatic nerves. RSC are cryopreserved either at primary or passage one culture and delivered frozen. Each vial contains >5 x 10^5 cells in 1 ml volume. RSC are characterized by immunofluorescence with antibodies specific to S-100, GFAP, and CD90. RSC are negative for mycoplasma, bacteria, yeast, and fungi. RSC are guaranteed to further culture under the conditions provided by Creative Bioarray.
Rat Schwann Cells are glial cells obtained from the peripheral nervous system of rats and are known to support axons, myelinate nerve fibers, and aid in nerve regeneration. Schwann cells are the major glial cells present in peripheral nerves and are critical for neuronal function and repair after injury. Rat Schwann cells are used as a well-characterized experimental model in various studies due to their excellent growth profile and high similarity to mammalian peripheral nerve biology.
Under optimal culture conditions rat Schwann cells maintain their spindle-shaped or elongated bipolar morphology while growing as adherent cells. They express typical Schwann cell markers, such as S100β, SOX10, p75 neurotrophin receptor, and myelin-associated proteins like myelin basic protein (MBP) and peripheral myelin protein 22 (PMP22), depending on their differentiation state. When supplied with appropriate growth factors, they exhibit stable proliferation and can be induced to myelinating or repair phenotypes.
Rat Schwann cells functionally support axonal survival and regeneration by secreting neurotrophic factors, extracellular matrix components, and cytokines. They are commonly employed in studies of peripheral nerve development, demyelination and remyelination, nerve injury, and neuroinflammation. Rat Schwann cells are also used as a model for studying Schwann cell-neuron interactions, myelin disorders, and peripheral neuropathies.
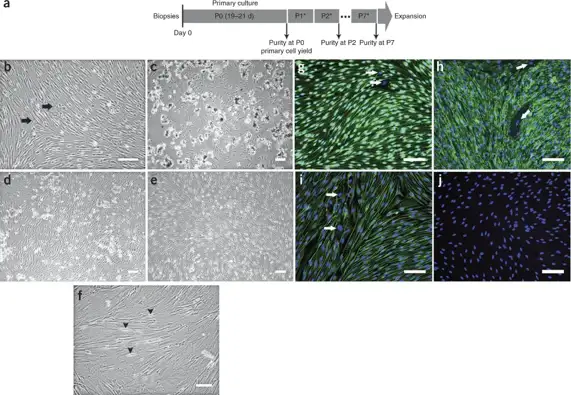
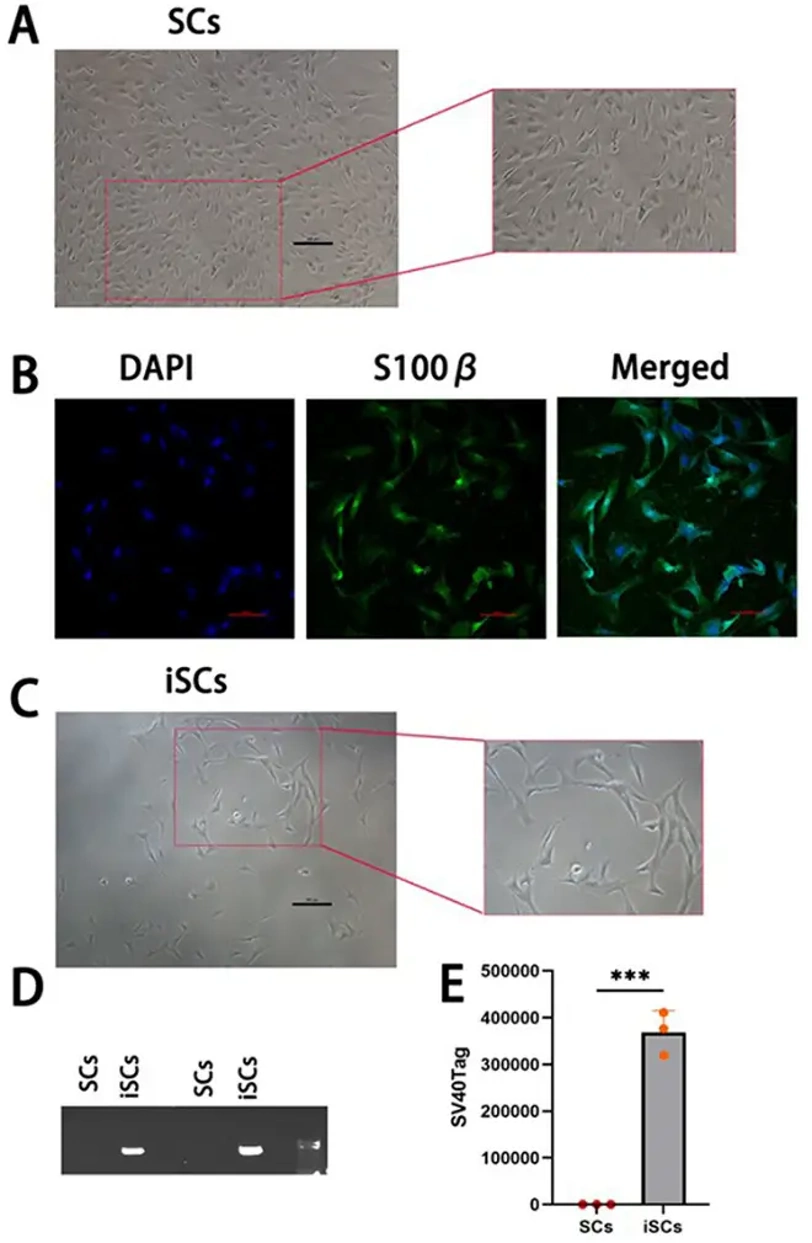
Comparison Of Primary Schwann Cells and Immortalised Schwann Cells
Primary Schwann cells (SCs) at passage 2 (P2) were transfected with SV40T-Ag and cultured for 10 generations. Hygromycin was used to select for an immortalized Schwann cell line (iSCs), which exhibited spindle-shaped morphology with slender processes (Fig. 1C). RNA was extracted from 12th generation iSCs and primary cells for agarose gel electrophoresis (Fig. 1D) and RT-qPCR analysis (Fig. 1E). Primary cells did not express SV40T-Ag, while iSCs stably expressed SV40T-Ag (P<0.01), indicating successful immortalization.
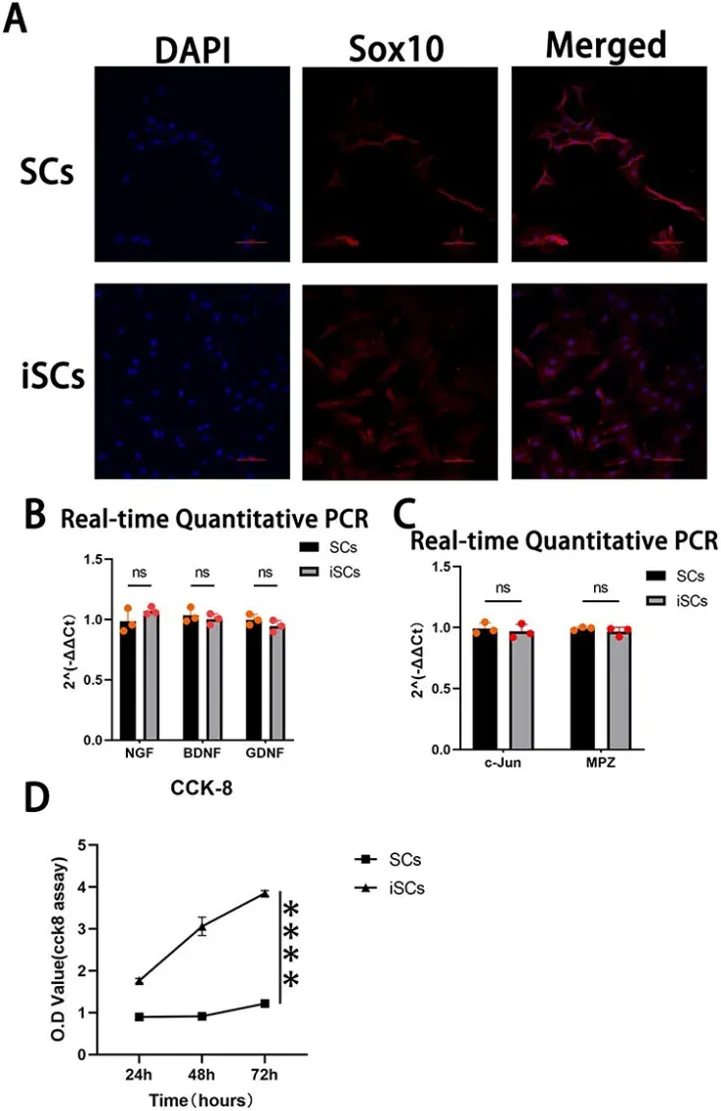
To detect the expression of SC-specific markers in iSCs, Sox10 was used as a marker related to SC maintenance and regeneration. Immunofluorescence staining showed red fluorescence in the cytoplasm and blue fluorescence in the nucleus of primary cells and iSCs (Fig. 2A). Analysis of NGF, BDNF, and GDNF gene expression by RT-qPCR did not reveal significant differences between primary SCs and iSCs (Fig. 2B). Similarly, there were no significant differences in the expression of two essential genes related to myelin formation, c-Jun and MPZ (Fig. 2C). In summary, iSCs are genotypically similar to primary SCs, which can be used for neural research. Proliferation of iSCs measured by CCK-8 assay revealed that iSCs had a significantly higher proliferation rate than primary SCs (Fig. 2D).
Ask a Question
Write your own review