Rat Bone Marrow Mononuclear Cells
Cat.No.: CSC-C4410X
Species: Rat
Source: Bone Marrow
Cell Type: Mononuclear Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat bone marrow mononuclear cells are derived from the normal bone marrow tissue of experimental rats. The bone marrow serves as the body's hematopoietic tissue and resides in multiple bones including the medullary cavities of long bones and the trabecular parts of flat bones. It consists of two primary forms which are red bone marrow and yellow bone marrow. Red bone marrow serves as the primary site for hematopoiesis since it houses numerous hematopoietic stem cells and progenitor cells. Yellow bone marrow mainly comprises fat cells and shows restricted ability to produce blood elements. Researchers commonly obtain rat bone marrow mononuclear cells directly from red bone marrow tissue.
BMNCs represent a mixed cell population composed of lymphocytes (including T cells and B cells), monocytes, hematopoietic stem cells, endothelial progenitor cells (EPCs), and additional cells. They have the ability to manage immune responses because they produce cytokines including IL-1β and TNF-α; they can differentiate into endothelial progenitor cells to promote angiogenesis; and they can migrate to damaged brain tissue to promote the proliferation and differentiation of neural stem cells, thereby improving neurological function after stroke.
Therefore, BM-MNCs demonstrate wide potential for use across regenerative medicine and immunotherapy fields. For example, BM-MNCs treat ischemic heart disease by enhancing blood flow in ischemic myocardial regions through the stimulation of angiogenesis. Bone defect repair and cartilage regeneration research utilizes BM-MNCs because they transform into osteoblasts and chondrocytes which stimulate tissue regeneration. BM-MNCs serve as tools for researching hematopoiesis along with immune system functions and the stem cell differentiation abilities.
PRPs Upregulated Proliferative Ability of BM MSCs
Cartilage defects often lead to osteoarthritis due to limited self-regeneration ability. Traditional bone marrow stimulation (BMS) techniques, such as multiple channeling, fail to generate hyaline cartilage, forming fibrocartilage instead. The restriction of endogenous bone marrow mesenchymal stem cells (BM MSCs) in number and strength contributes to this outcome. Research indicates that platelet-rich plasma (PRP) demonstrates potential benefits for tissue healing and increasing stem cell multiplication. Lee et al. investigated if second-generation PRP (2G PRP) with extended retention time improves cartilage repair through enhanced local proliferation and migration of BM MSCs to repair sites.
They isolated mononuclear cells from the bone marrow of femurs and tibias in 8-week-old SD rats. After subculturing adherent BM MSCs on the 10th day researchers used BM MSCs that were in passages 3 and 4 for this study. The influence of PRPs on BM MSC proliferation was evaluated by treating MSCs with 10% (v/v) PRP supernatant followed by measurements of proliferation across a 10-day period (Fig. 1A). BM MSC proliferation showed peak levels on days 3 and 5. On days 3 and 5 the 2G PRP group as well as the groups treated with PRP with Ca2? + THRB and PRP with Ca2? showed significantly greater growth compared to the control group. The MSC proliferation increased 5.96-fold, 5.68-fold, and 5.39-fold in the 2G PRP group and Ca2? + THRB and Ca2? groups respectively compared to the baseline measurement at day 0 after 5 days. The PRP group did not exhibit any growth improvements over the control group. The absence of soluble factors in washed-out PRP demonstrates its inability to stimulate cell growth. The 2G PRP treatment demonstrated the greatest proliferation enhancement at 1.72-fold over the PRP group on days 3 and 5, whereas Ca2? + THRB and Ca2? treatments also improved proliferation beyond PRP levels but showed no significant differences between them.
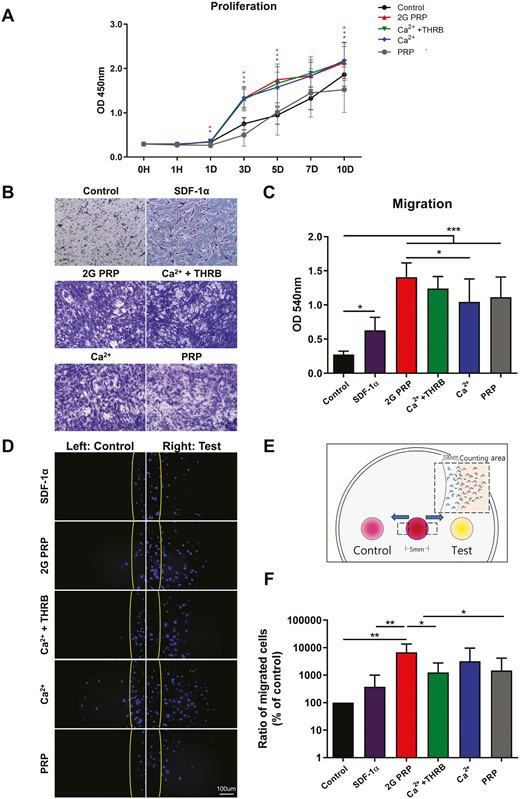 Fig. 1. Effects of 2G PRP on proliferation and migration of rat BM MSCs (Lee MJ, Jiang J, et al., 2024).
Fig. 1. Effects of 2G PRP on proliferation and migration of rat BM MSCs (Lee MJ, Jiang J, et al., 2024).
Isolation, Induction and Identification of NTCSCs
Yu's team investigated the efficacy of transplanting bone marrow neural tissue-committed stem cell-derived sensory neuron-like cells for repairing peripheral nerve sensory impairments in rats. SD rat bone marrow mononuclear cells were isolated and cultured as previously described, and cell spheres were obtained through single-cell cloning and serum-free suspension culture (Fig. 2a). After culturing to the third generation, neuronal cells were induced from neural tissue-committed stem cells (NTCSCs) (Fig. 2b). The NTCSCs exhibited neuron-like morphology, with cytoplasm retracting to form round cells with a typical perikaryon shape, enhanced stereoscopic sensation, and refraction. The cells had 2 to 5 elongated protrusions resembling neuronal axons, with dendrite-like branches at their ends. These protrusions intertwined to form a network. Immunofluorescence staining was performed on the induced and differentiated cells for neuronal markers NeuN, MAP-2, and GluR4. Results showed that NeuN, MAP-2, and GluR4 were expressed in the induced cells (Fig. 3). MAP-2 and NeuN fluorescence was red, with MAP-2 mainly in the cytoplasm and NeuN in both the nucleus and cytoplasm. GluR4 fluorescence was green, with most expression in the cytoplasm and a small amount on the cell membrane. DAPI staining of the nucleus was blue.
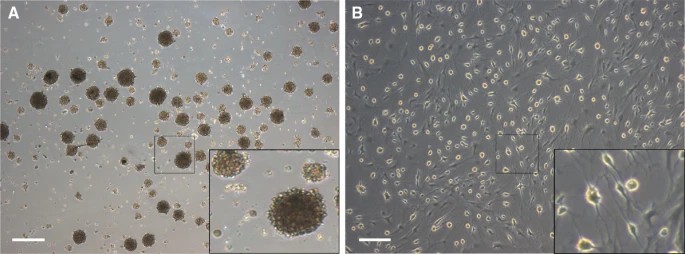 Fig. 2. Morphological characteristics of cell spheres and neuron-like cells (Yu Z, Xu N, et al., 2019).
Fig. 2. Morphological characteristics of cell spheres and neuron-like cells (Yu Z, Xu N, et al., 2019).
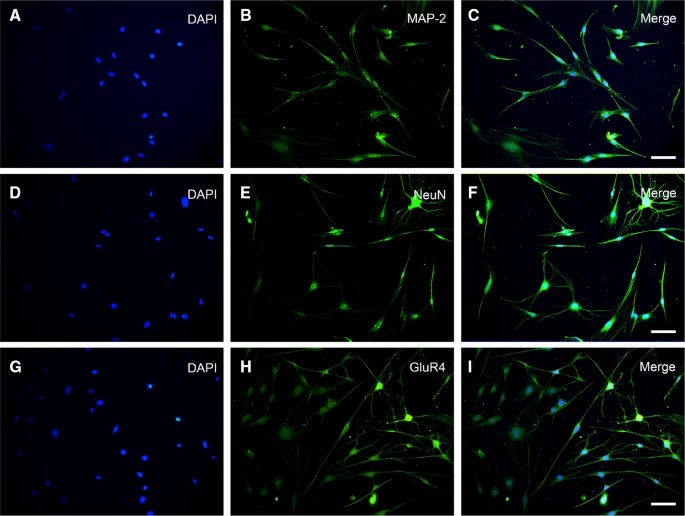 Fig. 3. Characteristic proteins of NeuN, MAP-2, and GluR4 were expressed in the cells (Yu Z, Xu N, et al., 2019).
Fig. 3. Characteristic proteins of NeuN, MAP-2, and GluR4 were expressed in the cells (Yu Z, Xu N, et al., 2019).
Ask a Question
Write your own review