Human Small Airway Epithelial Cells
Cat.No.: CSC-C4106X
Species: Human
Source: Lung; Airway
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Cell Features:
HBTEC are cryopreserved as primary cells. Cells are isolated from human bronchi/trachea and expanded once in culture vessels before being harvested for cryopreservation.
HSAEC are cryopreserved after primary cells are isolated from human lung tissue and expanded once in culture vessels before being harvested for cryopreservation.
Creative Bioarray's Human Airway Epithelial Cells are not exposed to retinoic acid during isolation or expansion.
Human Airway Epithelial Cells are extensively tested for quality and optimal performance.
Creative Bioarray guarantees performance and quality.
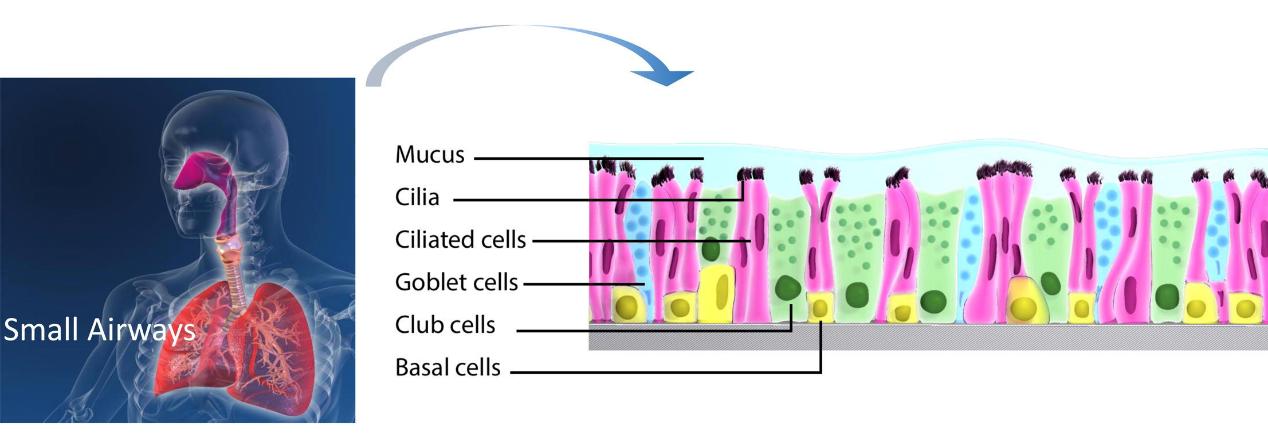
The small airways are located at the interface between alveoli and conducting airways. Airway epithelial cells form a continuous lining within the airways. They play a unique role as a protective physical and functional barrier against external deleterious agents.
Human Small Airway Epithelial Cells (HSAEpC) are isolated from the distal portion of lung tissue in the bronchiole area of the respiratory tract. They participate in host defense by producing chemokines and expressing adhesion molecules, thereby regulating immune responses. They also produce liquids contributing to pulmonary fluid balance. Many airway diseases, such as asthma, bronchiolitis, chronic obstructive pulmonary disease, and cystic fibrosis, involve damage to the airway surface epithelium. The study of human SAEpC may help to identify new therapeutic options for preventing airway disorders. HSAEpC are also useful tools for establishing in vitro disease models for high-throughput and high-content screening.
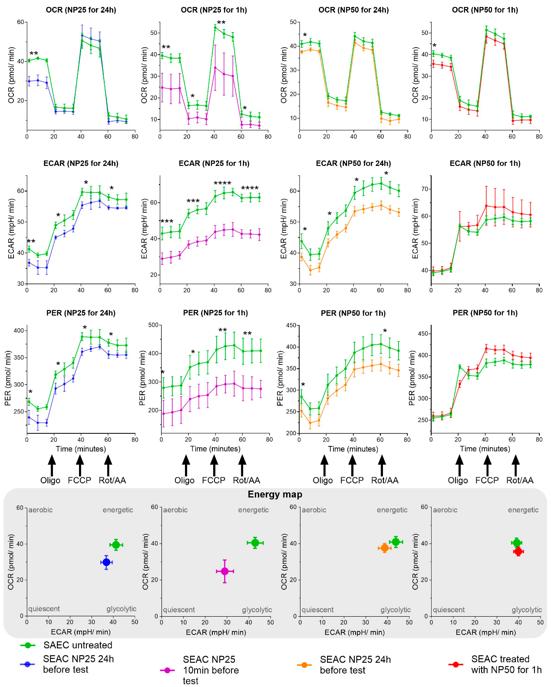
Nanoplastics Penetrate Human Bronchial Smooth Muscle and Small Airway Epithelial Cells and Affect Mitochondrial Metabolism
Micro- and nanoplastic particles, including common forms like polyethylene and polystyrene, have been identified as relevant pollutants, potentially causing health problems in living organisms. The mechanisms at the cellular level largely remain to be elucidated. This study aims to visualize nanoplastics in bronchial smooth muscle (BSMC) and small airway epithelial cells (SAEC), and to assess the impact on mitochondrial metabolism.
Healthy and asthmatic human BSMC and SAEC in vitro cultures were stimulated with polystyrene nanoplastics (PS-NPs) of 25 or 50 nm size, for 1 or 24 h. Live cell, label-free imaging by holotomography microscopy and mitochondrial respiration and glycolysis assessment were performed. Furthermore, 25 and 50 nm NPs were shown to penetrate SAEC, along with healthy and diseased BSMC, and they impaired bioenergetics and induce mitochondrial dysfunction compared to cells not treated with NPs, including changes in oxygen consumption rate and extracellular acidification rate.
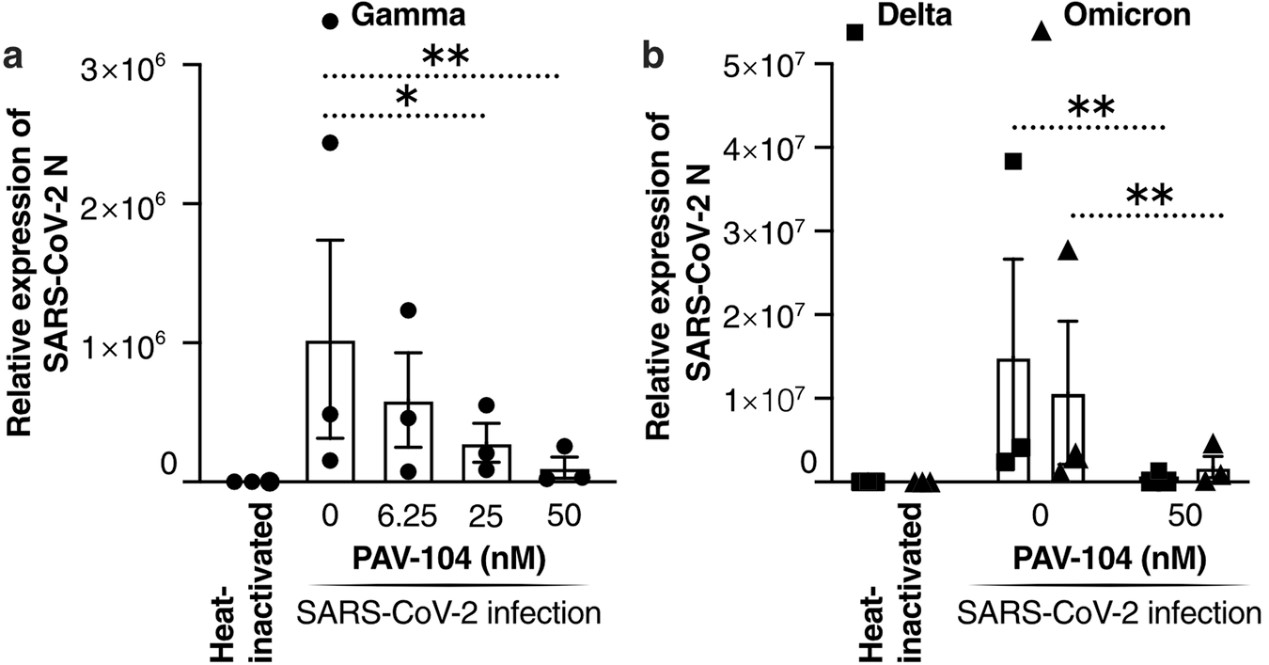
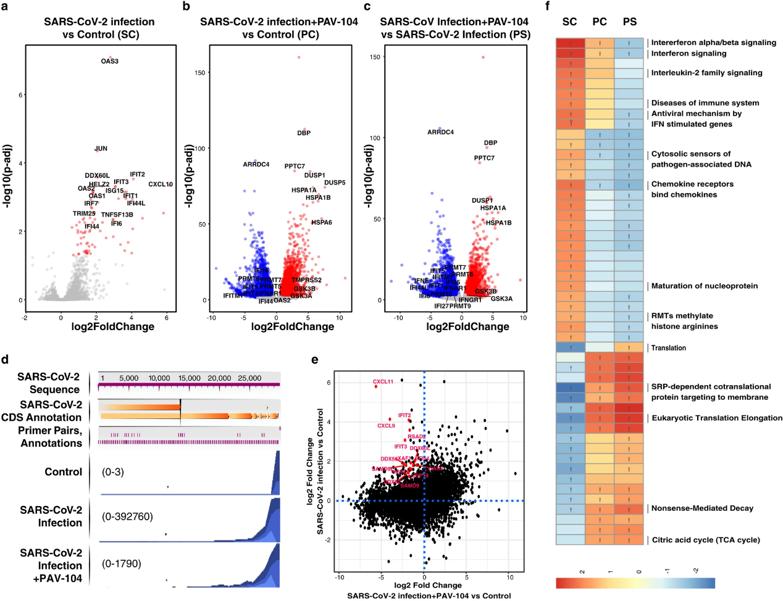
A Viral Assembly Inhibitor Blocks SARS-CoV-2 Replication in Airway Epithelial Cells
The ongoing evolution of SARS-CoV-2 to evade vaccines and therapeutics highlights the need for innovative therapies with high genetic barriers to resistance. Therefore, there is pronounced interest in identifying new pharmacological targets in the SARS-CoV-2 viral life cycle.
PAV-104, a small molecule identified through a cell-free protein synthesis and assembly screen, was recently shown to target host protein assembly machinery in a manner specific to viral assembly. This study investigated the capacity of PAV-104 to inhibit SARS-CoV-2 replication in human airway epithelial cells (AECs). Results showed that PAV-104 inhibits >99% of infection with diverse SARS-CoV-2 variants in immortalized AECs, and in primary human AECs cultured at the air-liquid interface (ALI) to represent the lung microenvironment in vivo. PAV-104 interacts with SARS-CoV-2 nucleocapsid (N) and interferes with its oligomerization, blocking particle assembly. Transcriptomic analysis reveals that PAV-104 reverses SARS-CoV-2 induction of the type-I interferon response and the maturation of nucleoprotein signaling pathway known to support coronavirus replication. PAV-104 is a promising therapeutic candidate for COVID-19 with a mechanism of action distinct from existing clinical management approaches.
Ask a Question
Write your own review