Human Cerebellar Granule Cells (HCGC)
Cat.No.: CSC-7811W
Species: Human
Source: Brain
Cell Type: Granule Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Among brain neurons, human cerebellar granule cells (HCGC) stand out as one of the most numerous types. HCGC develop mainly during the embryonic stage of the cerebellum via differentiation of neural progenitor cells found in the rhombic lip. In the developmental phase these cells move toward the internal granule cell layer (IGL) of the cerebellar cortex. HCGC neurons display typical morphology with multiple short dendrites alongside a long axon. When cultured in vitro these cells develop intricate neural networks that display advanced neuronal characteristics including dense neural fiber formations and excitatory amino acid receptor expression.
The cerebellum's physiological functions depend significantly on the role of HCGC cells. These neurons send signals through glutamatergic synapses to contribute to cerebellar motor coordination and balance control functions as well as cognitive processes. Granule cells maintain cerebellar neural signaling functionality through their synaptic connections with Purkinje cells. Additionally, the development of the cerebellum depends on granule cells which drive the expansion and specialization as well as movement of neural progenitor cells.
Due to their abundance, ease of culture, and typical neuronal characteristics, HCGC are widely used in neuroscience research. Researchers utilize these cells as model systems to explore neurodevelopment processes and investigate neurodegenerative diseases like Parkinson's and Alzheimer's disease alongside neuroprotective strategies. Moreover, granule cells are used to investigate neuronal survival, apoptosis, and activity-dependent mechanisms.
Euxanthone Inhibits Medulloblastoma Proliferation by Suppressing RANK/RANKL Pathway
The most frequent pediatric brain tumor is medulloblastoma which presents a 5-year survival rate that remains under 70%. The standard treatments for medulloblastoma including surgical intervention alongside chemotherapy and radiation therapy frequently result in major negative effects such as cognitive decline and the development of secondary tumors. The plant-derived flavonoid euxanthone extracted from Polygala caudate has demonstrated anticancer capabilities in several types of cancer. Xu's team plans to investigate how euxanthone impacts medulloblastoma cells through the RANK/RANKL pathway.
The research tested human medulloblastoma cells (D425) and human cerebellar granule cells (HCGC) with different concentrations of euxanthone ranging from 0 to 160 μM to determine cell survival using the CCK-8 assay. The IC50 value of 145 μM determined the dose-dependent decrease in cell viability of HCGC cells (Fig. 1A). Human medulloblastoma D425 cells displayed greater sensitivity to euxanthone treatment with an IC50 value of 18 μM (Fig. 1B). The exposure of D425 cells to 0, 5, 10, or 20 μM euxanthone resulted in dose-responsive suppression of RANK, RANKL, and OPG gene expression (Fig. 1C–E). The data demonstrates that euxanthone selectively reduces medulloblastoma cell viability through suppression of the RANK/RANKL signaling pathway.
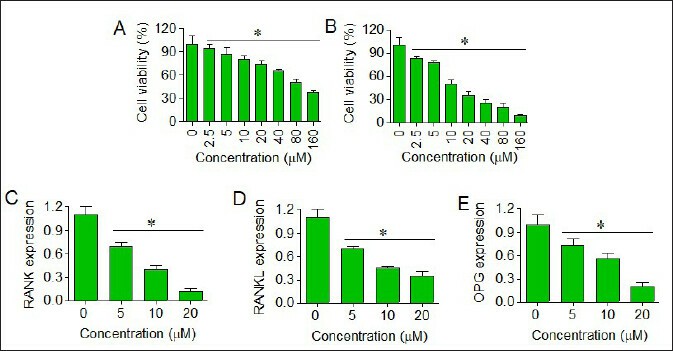 Fig. 1. Morphology of theSUP- HD1 cell line (Naumovski L, Utz P, et al., 1990).
Fig. 1. Morphology of theSUP- HD1 cell line (Naumovski L, Utz P, et al., 1990).
Moringa oleifera Seed Ethanol Extract and Its Active Component Kaempferol Potentiate Pentobarbital-Induced Sleeping Behaviours in Mice via a GABAergic Mechanism
Sleep disorders, including insomnia, affect a significant portion of the population, leading to daytime dysfunction and decreased immunity. Current pharmacological treatments often cause side effects such as drug dependence and cognitive impairment. Moringa oleifera (MO) is a plant with high nutritional and medicinal value, but its effects on sleep are unclear.
Liu's team investigated whether MO seed ethanol extracts (EEMOS) could improve sleep and explore the underlying mechanisms. Results showed that EEMOS (2 g/kg) and kaempferol (2 mg/kg) significantly enhanced sedative-hypnotic effects, increased γ-aminobutyric acid levels, and upregulated GAD65 and GABAA receptor α1-subunit expression in mice, suggesting that EEMOS improves sleep via the GABAA-ergic system. In the GABAergic system, the extracellular Cl– that moves into the human cerebellar granule cells (HCGC) was measured by MQAE. The results were denoted by relative fluorescence F0/F ratio, where F was the fluorescence as a function of each sample and F0 was the fluorescence without Cl– ions. The F0/F ratio was directly proportional to intracellular Cl– concentration. As shown in Figure 2, compared to control group, a significant increase in Cl– influx was shown in the cells treated with EEMOS (4 μg/mL, p < 0.05; 8 μg/mL, p < 0.01; Fig. 2A) and KA (8 μg/mL, p < 0.01, Fig. 2B). PENT (10 mM) that acts as a positive control also increased the influx of Cl– in HCGC cells (p < 0.01).
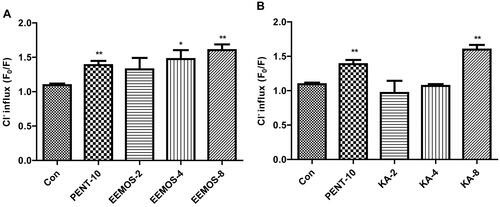 Fig. 2. Effect of EEMOS on Cl– influx in HCGC cells (Liu WL, Wu BF., et al., 2022).
Fig. 2. Effect of EEMOS on Cl– influx in HCGC cells (Liu WL, Wu BF., et al., 2022).
Ask a Question
Write your own review