Human Neurons-hippocampal (HN-h)
Cat.No.: CSC-7751W
Species: Human
Source: Brain
Cell Type: Neuron
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The human Neurons-hippocampal (HN-h) cell line functions as an essential platform for research into neuronal damage and treatment methods because the hippocampus is central to memory formation and learning processes. The dentate gyrus, parahippocampal gyrus and hippocampal cornu are among the structures within this complex brain region. The structure of hippocampal neurons which includes pyramidal cells with distinct dendrites and axons provides valuable knowledge about neurodegenerative diseases such as Alzheimer's Disease (AD). For example, the elevated levels of c-fos mRNA in HN-h cells from AD models demonstrate neuronal stress while modulation of genes such as SOX7 highlights cellular reactions to oxidative damage and injury. Neurotoxicity research utilizes these cells to examine the destructive effects of toxins that produce glutamate-induced ROS which causes neuronal death but can be reduced by antioxidants such as L-serine. Furthermore, HN-h cells play a crucial role in studying neuroprotective and repair mechanisms because D-galactose defends these cells by lowering mitochondrial membrane potential and oxidative stress while also reducing β-amyloid accumulation which marks AD pathology.
High Glucose Promotes tau Hyperphosphorylation by Inhibiting the Expression of m6A Demethylase ALKBH5 in HN-h Cells
Elevated blood glucose in diabetes leads to tau hyperphosphorylation in hippocampal neurons, causing cognitive decline. m6A modifications affect cognitive functions and are linked with neurodegenerative conditions. ALKBH5, a m6A demethylase, is pivotal in neurodevelopment and potentially in neurodegeneration through its regulation of m6A marks. Qu's team aims to uncover the mechanism by which high glucose induces tau hyperphosphorylation via ALKBH5-mediated effects on Dgkh mRNA in hippocampal neurons, exploring potential therapeutic targets to reverse diabetic cognitive dysfunction.
Human Neurons-hippocampal (HN-h) cells were exposed to various high glucose (HG) concentrations (25, 33, 50, and 75 mM) to identify a condition that would not affect viability. A significant viability reduction was observed with 75 mM for 2 days and 50 mM for 3 days (Fig. 1A and B). Osmolarity control didn't significantly affect viability. Thus, 50 mM glucose for 2 days was selected for further experiments.The HG group showed significant decreases in ALKBH5 mRNA and protein levels compared to control samples (Fig. 1C and D). The levels of METTL3, METTL14, and FTO proteins remained unchanged. HG treatment significantly increased p-tau levels at T231 and PHF-1 epitopes (Fig. 1D), suggesting that HG induces tau hyperphosphorylation alongside reduced ALKBH5 expression in HN-h cells. To confirm ALKBH5's role in tau phosphorylation, HN-h cells were infected with a lentivirus carrying ALKBH5, reversing HG-induced tau hyperphosphorylation (Fig. 1E and F). The silencing of ALKBH5 using siRNA produced a slight elevation in p-tau levels at T231 and PHF-1 in comparison to control and showed more pronounced results than HG plus scrambled siRNA (Fig. 1G and H). Research demonstrates high glucose levels lead to reduced ALKBH5 expression which triggers tau protein hyperphosphorylation.
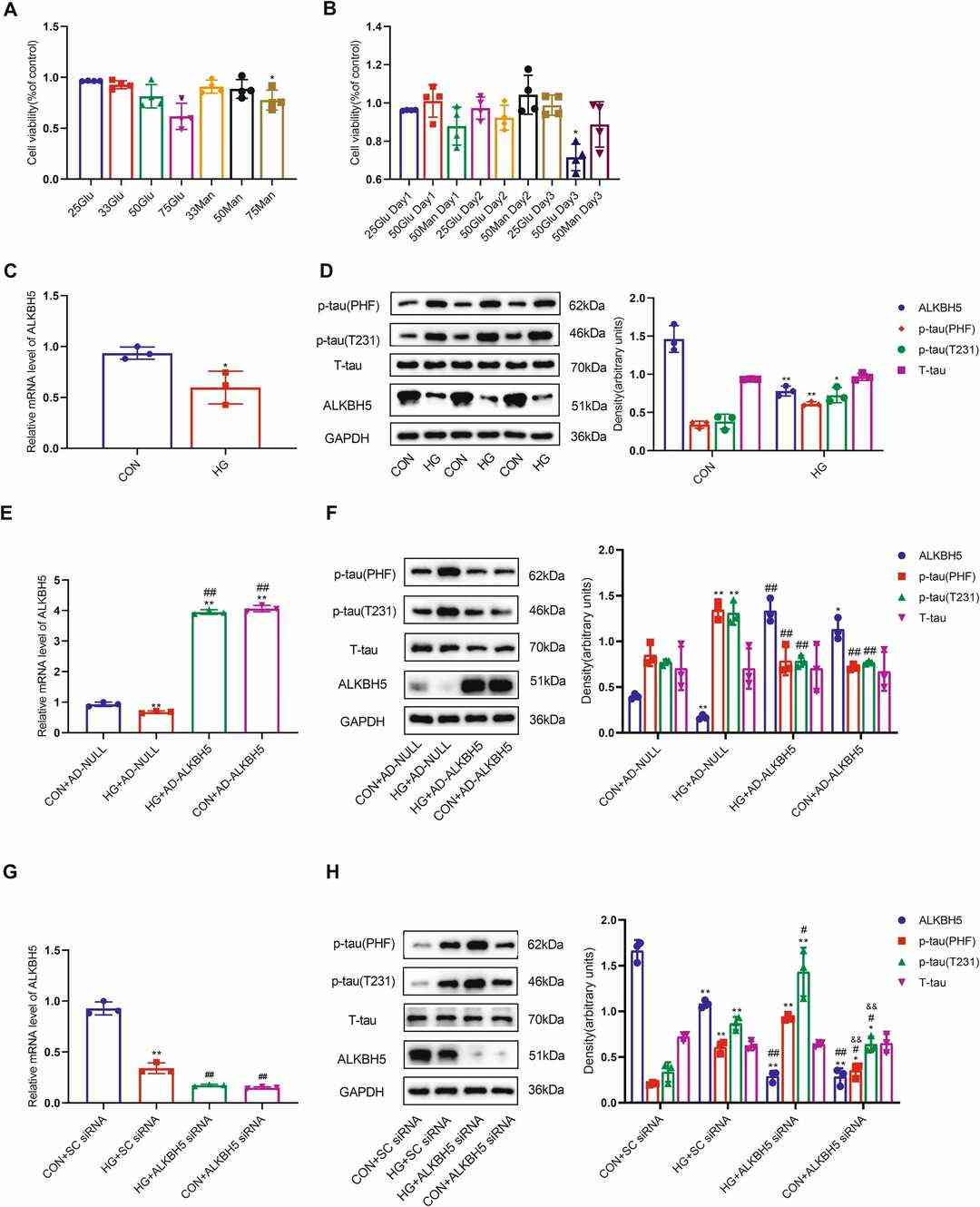 Fig. 1. ALKBH5 downregulation leads to tau hyperphosphorylation in HN-h cells (Qu M, Zuo L, et al., 2023).
Fig. 1. ALKBH5 downregulation leads to tau hyperphosphorylation in HN-h cells (Qu M, Zuo L, et al., 2023).
Upregulation of miR-223-3p Expression is a Mechanism Involved in the Neuroprotective Effect of DEX Against H2O2 Induced-cytotoxicity
Dexmedetomidine (DEX) is an anesthetic known for its neuroprotective capabilities. It is used for sedation in medical procedures and has shown protection against various organ ischemia. Recent studies have linked its neuroprotection to modulation of specific pathways involving apoptotic and oxidative stress signaling, as well as inflammation. MicroRNAs (miRNAs) play roles in neuroprotection through regulation of cellular stress responses.
Wang's team investigated a potential implication of microRNA (miR)?223?3p in the DEX?induced anti?oxidative effect on neuronal cells. MTT assay indicated that DEX protects HN-h cells against H2O2-induced cytotoxicity. To investigate miR-223-3p's involvement in DEX protection against H2O2-based cell damage HN-H cells received miR-223-3p mimic or inhibitor treatment before H2O2 exposure. The RNA mimic resulted in higher miR-223-3p levels and the inhibitor led to lower miR-223-3p levels. MTT assays and flow cytometry outcomes displayed that the inhibitor increased both cellular death and apoptotic activity while the mimic showed protective effects against these outcomes (Fig. 2A and B). The activation of miR-223-3p through mimics reduced LDH leakage together with MDA, ROS, calcium ion levels and CAMII phosphorylation which indicates that miR-223-3p serves as a neuroprotective agent (Fig. 2C-H).
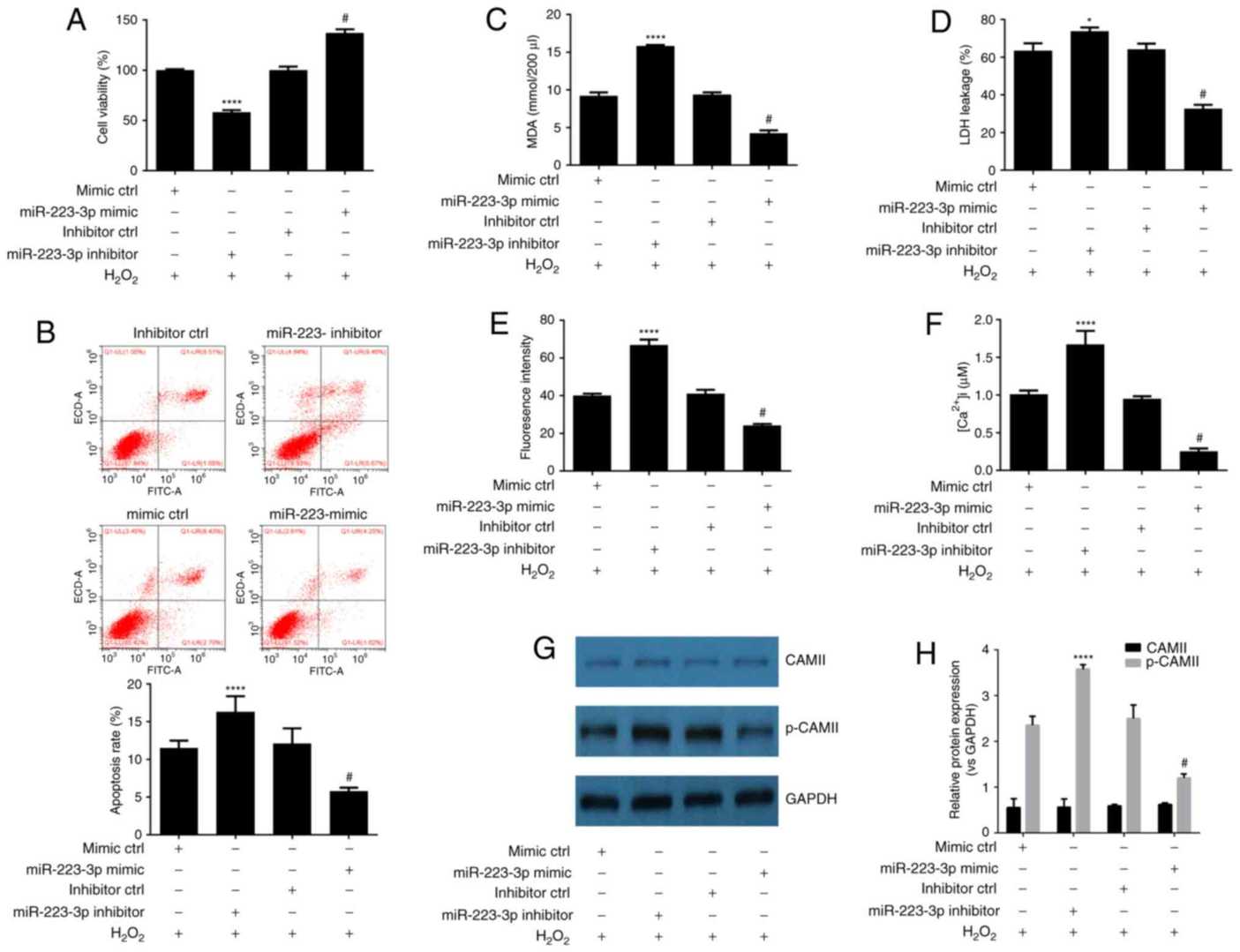 Fig. 2. Upregulation of miR-223-3p expression is a mechanism involved in the neuroprotective effect of DEX against H2O2 induced-cytotoxicity (Wang Q, Yu H, et al., 2018).
Fig. 2. Upregulation of miR-223-3p expression is a mechanism involved in the neuroprotective effect of DEX against H2O2 induced-cytotoxicity (Wang Q, Yu H, et al., 2018).
Ask a Question
Write your own review