Human Cardiac Progenitor Cells
Cat.No.: CSC-C3118
Species: Human
Source: Heart
Cell Type: Progenitor Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Cardiac progenitor cells may be used in various in vitro, or in vivo studies; transplantation into normal or diseased systems; cardiac toxicology studies; or cardiovascular developmental studies.
Cardiovascular disease (CVD) could affect cardiac tissue and the circulatory system. According to data released by the American Heart Association, CVD is considered the leading cause of death worldwide, accounting for more than 17.3 million deaths every year. By 2030, this number is thought to be reached to more than 23.6 million people. Up to present, some therapeutic strategies have been developed to only to eliminate the clinical symptoms, but the application of novel alternative treatment for CVD remains to be answered.
It is well known that CVD itself could have detrimental effects on cardiomyocytes by inducing cytotoxicity, particularly apoptosis, autophagy, and necrosis. Recently, regenerative medicine has introduced new ways to facilitate the restoration of damaged tissues and organs in CVD example. Based on scientific opinion, stem cells (SCs) have potential to multipotentiality, self-renewability and giving rise to different lineages, and can potently differentiate into different functional cell types which makes them as suitable candidate for the regeneration of various organs, peculiarly cardiac tissue.
Cardiac progenitor cells (CPCs) are endogenous cardiac SCs that found to express tyrosine kinase receptors, c-Kit and other stemness features in adult heart, contributing to the regeneration of cardiac tissue after injury. This lineage is able to efficiently trans-differentiate into different cell types such as cardiomyocytes, endothelial cells, and smooth muscle cells. After the introduction of CPCs, the theory that cardiac cells were unable to regenerate cardiac tissue gradually disappeared and thereby many researchers began to determine the possibility of their experimental and clinical usage as a therapeutic agent.
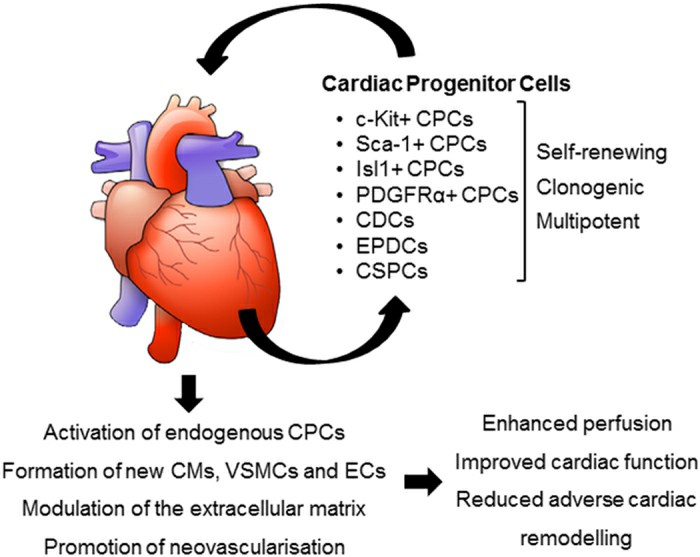 Fig. 1. Potential mechanisms of action of transplanted CPCs (Le, T. Y. L. and J. J. H. Chong. 2016).
Fig. 1. Potential mechanisms of action of transplanted CPCs (Le, T. Y. L. and J. J. H. Chong. 2016).
Based on released data, transplanted CPCs exerted its therapeutic effects through different mechanisms. They could potentially tans-differentiate into cardiac and endothelial lineages. The exogenous CPCs, in turn, activate endogenous resident progenitor cells. Dynamic regulation of the extracellular matrix (ECM) and appropriate cardiomyocytes fusion begins after CPCs transplantation. CPCs also have potency to release a variety of factors via paracrine manner. For example, it was revealed that CPCs-derived exosomes could enhance anti-apoptotic, immunomodulatory or pro-angiogenic situation.
Isolation and Culture of CPCs
CPCs were isolated from the right atrial appendage (RAA) and left ventricle (LV) through cell dissociation using collagenase II. Due to the variation in size between RAA and LV samples, cells were seeded onto an appropriately sized plate or flask based on the weight of the tissue to minimize differences in initial seeding density. The resultant CPCs were characterized by flow cytometry for the expression of mesenchymal stem cell (MSC) markers, CD90 (thymocyte differentiation antigen, Thy-1) and CD105 (endoglin), as well as the circulating hematopoietic progenitor cell marker CD34.
Majority of the cells were negative for CD34 population, although due to the ischemic nature of the tissue collected, they still expressed CD34, which was especially more in LV CPCs (18.3% ± 3.3% in RAA CPCs vs. 24.7% ± 6.0% in LV CPCs, P = 0.23, n = 6 patients, Fig. 1A, C). CD34 negative population were then used to measure the expression of MSC markers, CD90 and CD105. CD90 expression was significantly higher in RAA CPCs compared to LV CPCs (78.7% ± 5.9% in RAA CPCs vs. 54.4% ± 11.0% in LV CPCs, P = 0.03, n = 6 patients, Fig. 1B, D). CD105 expression was high in both RAA CPCs and LV CPCs, with nearly all cells expressing the cell marker (99.%0 ± 0.6% in RAA CPCs vs. 99.3% ± 0.1% in LV CPCs, P = 0.2505, n = 6 patients, Fig. 1B, D). Further, immunocytochemical analysis confirmed the expression of cardiac transcription factor Islet-1 in the undifferentiated CPCs (Fig. 1E).
CPCs grown in differentiation medium showed positive staining for cardiomyocyte-specific markers MHC (Fig. 1F) and connexin-43 (Fig. 1G). RT-PCR analysis confirmed the expression of cardiac troponin and α-sarcomeric actin in differentiated CPCs (Fig. 1H). As expected, there was no difference in the differentiation potential between RAA and LV CPCs.
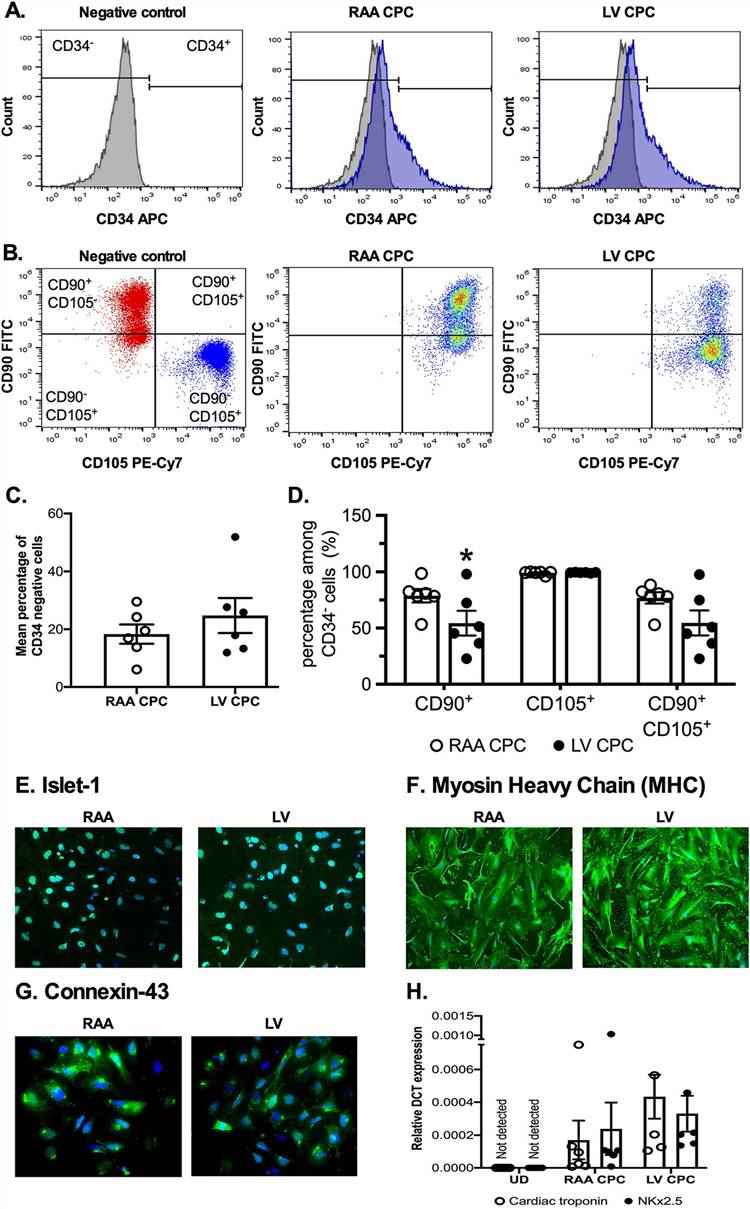 Fig. 1. Comparable expression of cell surface markers between RAA CPCs and LV CPCs (McQuaig, Ryan, et al., 2020).
Fig. 1. Comparable expression of cell surface markers between RAA CPCs and LV CPCs (McQuaig, Ryan, et al., 2020).
Deep Comparative Transcriptome Analysis of CPCs by mRNA Sequencing
To define specific CPCs functions, human CPCs from three independent donors (CPC1-3) were compared to human bone marrow MSCs (aiming to identify putative genes related to multipotency) using mRNAseq, with human dermal fibroblasts (HDFs) as a distant reference (to discard genes expressed similarly in all cell types). After a preliminary evaluation of the impact of different culture media in CPCs vs. HDFs growth, we selected to culture each cell type in its optimal media.
CPCs, MSCs and HDFs were compared (FDR < 0.05) only for coding genes from total and differentially expressed gene (DEG) data, using replicates and/or technical duplicates of all samples at the indicated passages. CPCs mRNAseq data rendered 12,242 protein-coding genes. Normalized heat map and cluster analysis confirmed CPCs, MSCs and HDFs as cell lineages significantly different from each other (Fig. 2a, b). In addition, we confirmed that the expression profiles were not significantly affected by culture passage, as only 167 out of 11,767 total genes analyzed showed significant variations with passages.
Comparative analysis found 2,096 DEG (17.8%) for the CPCs/MSCs comparison (p.adj values < 0.05; Fig. 2c), with 1,003 highly preferentially expressed in CPCs by simultaneous comparison with HDFs (Fig. 2c). No significant differences were found in association with subcellular compartments. The majority of the top 10 upregulated genes in CPCs were specific for the CPCs/MSCs comparison (Fig. 2d) and were not found upregulated in the CPCs/HDFs comparison. Among the genes upregulated, C-X-C motif chemokine ligand 6 (CXCL6) and matrix metallopeptidase 1 (MMP1) showed the maximal differences (Fig. 2d). Other genes of interest not included among the most upregulated genes, such as GATA binding protein 4 (GATA4), calcium voltage-gated channel auxiliary subunit gamma 7 (CACNG7), G protein-coupled receptor 4 (GPR4) and cadherin 5 (CDH5), were similarly upregulated in CPCs relative to MSCs and HDFs (Fig. 2d). Aggrecan (ACAN) and cartilage oligomeric matrix protein/ thrombospondin-5 (COMP) were more clear examples of down-regulated transcripts in CPCs when compared with MSCs (Fig. 2d).
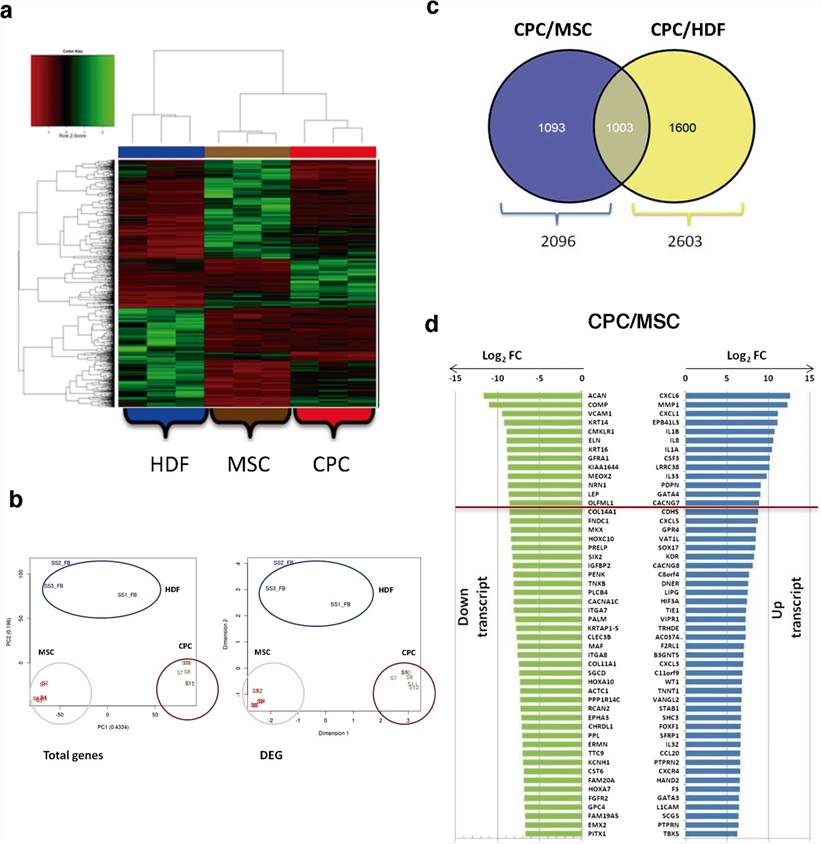 Fig. 2. RNAseq analysis of CPCs compared with MSCs and HDFs (Torán, José Luis, et al., 2019).
Fig. 2. RNAseq analysis of CPCs compared with MSCs and HDFs (Torán, José Luis, et al., 2019).
The types of cardiac progenitor cells (CPCs) in the adult heart include epicardium-derived cells, side population cells, cardio sphere-derived cells, c-Kit+ CPCs, cardiac colony forming unit fibroblasts (cCFU-F), Islet-1+ CPCs, and Sca 1+ CPCs.
Cardiac progenitor cells (CPCs) are able to proliferate and have the capacity to differentiate into cardiomyocytes. Thus, these cells are attractive targets in drug development applications for regenerative medicine programs focused on developing myocardial infarction and congestive heart failure treatments. They are also ideal for identifying and helping to avoid drug-mediated cardiac developmental toxicity problems across multiple programs, an application for which scientists require consistent and dependable access to human CPCs.
Check all containers for leakage or breakage. Directly and immediately transfer the cells from dry ice to liquid nitrogen and keep the cells in liquid nitrogen until they are needed for experiments.
It is recommended to use SuperCult® Progenitor Cell Medium (cat# CM-1074X) for the culturing of Human Cardiac Progenitor Cells.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Reliable products
The cells I received were of high quality and greatly helped my research endeavors.
12 Jan 2023
Ease of use
After sales services
Value for money
Write your own review

