
Human Cardiac Fibroblasts
Cat.No.: CSC-7818W
Species: Human
Source: Heart
Morphology: Fibroblasts
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can primary cells be kept at -20 °C.
Cardiac fibrosis is associated with almost all forms of cardiovascular disease, negatively impacting disease progression and clinical outcomes. This condition is characterized by excessive deposition of extracellular matrix (ECM) proteins, leading to scar formation and impairment of cardiac function. As for many fibrotic conditions, there are still no effective therapies to treat cardiac fibrosis.
Cardiac fibroblasts (CFs) are key cellular effectors in the development of cardiac fibrosis. When quiescent CFs convert into activated CFs, also known as myofibroblasts, these cells acquire a highly proliferative and secretory phenotype, producing excessive amounts of ECM proteins responsible for limiting cardiac function. Myofibroblasts can be derived from different cell sources, including fibroblasts, mesenchymal stem cells, endothelial cells, and immune cells. However, in the heart, the major source of myofibroblasts is CFs. Despite the substantial progress in our understanding of the mechanisms of CFs activation, the number of actionable targets in cardiac fibrosis remains limited. The lack of sustainable CFs sources and reliable cell culture methods have hindered the identification of putative targets and drug development studies.
Human cardiac fibroblasts (HCFs) from Creative Bioarray provide an excellent model system for studying many aspects of human heart function and pathophysiology. Our HCFs include aortic adventitial fibroblasts isolated from the tunica external of the ascending or descending aorta and cardiac fibroblasts isolated from the atrium or ventricle. Cells are isolated from normal or diseased adult heart tissue. Cryopreserved cells are shipped at passage 2.
Characterization of CFs
Human fetal CFs were cultivated onto 10 cm-diameter dishes for initial expansion. CFs exhibited a flat and spindle-shaped morphology typical of the fibroblastic phenotype (Fig. 1A). CFs strongly expressed fibroblast markers: CD90 in 97.36 ± 0.14% of cells, vimentin in 98.70 ± 0.06%, α-SMA in 97.19 ± 0.15%, DDR2 in 99.62 ± 0.04%, fibronectin in 97.47 ± 0.04%, and pan-cadherin in 96.06 ± 0.15% (n = 3). Conversely, very few cells expressed cardiomyocyte markers cTnT or α-actinin (2.52 ± 1.33% and 1.35 ± 0.40%, respectively; n = 3), ruling out the presence of cardiomyocytes in this population (Fig. 1B).
Additional characteristics of CFs were defined by high-throughput cell profiling for the co-expression of CD90 and various MSC markers: integrin subunit beta 1 (CD29), CD44, 5'-nucleotidase ecto (CD73), endoglin (CD105), vascular cell adhesion molecule 1 (VCAM1, CD106), PDGF receptor alpha (CD140A), STRO-1, and activated leukocyte cell adhesion molecule (CD166). The expressions of a set of MSC-negative markers (i.e., CD34, CD11b, CD19, CD45, and HLA-DR) and c-Kit (CD117) was also assessed. Although CFs shared some characteristics with MSCs, they presented a specific profile in which MSC markers such as CD140A and STRO-1 were not detected (Fig. 1C).
Similarly, the expression of CSC markers (i.e., ATP-binding cassette subfamily G member 2 (CD338), NK2 homeobox 5 (NKX2.5), ISL LIM homeobox 1 (Isl1), and GATA-binding protein 4 (GATA4)) was evaluated. CFs are also positive for most of the CSC markers but not for CD338. CFs also shared characteristics with CSCs, but the lack of expression of critical CSC markers such as CD338 in CFs distinguished the two lineages. Additionally, the expression of several markers of EPDCs (i.e., CD46, WT1 transcription factor (WT1), T-box 18 (TBX18), and myocyte enhancer factor 2C (MEF2C)) was evaluated, and CFs presented an EPDC-like profile. CFs also expressed some endothelial markers such as intercellular adhesion molecule 1 (CD54), but other endothelial markers were expressed over various patterns: platelet and endothelial cell adhesion molecule 1 (CD31) was detected over a broad intensity range, and VE-cadherin (CD144) was not detected (Fig. 1C).
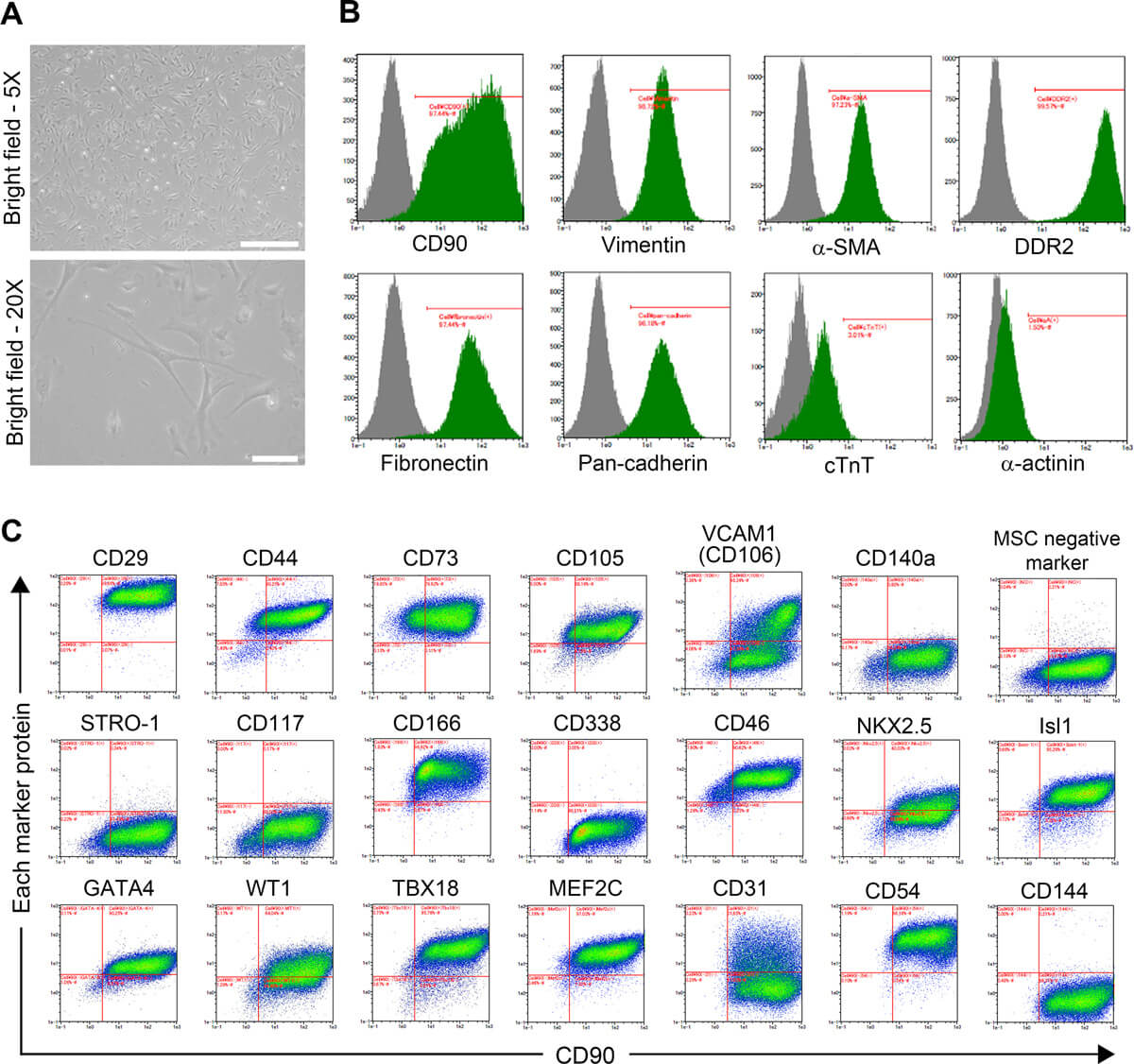 Fig. 1. Micrographs and immunoprofiling of CFs (Iwamiya, Takahiro, et al., 2020).
Fig. 1. Micrographs and immunoprofiling of CFs (Iwamiya, Takahiro, et al., 2020).
Cytokine and Chemokine Levels in Human Primary Cardiac Fibroblasts Cultured under Inflammatory Conditions
Human cardiac fibroblasts were subjected to hypoxic and LPS treatment respectively. Levels of cytokines and chemokines were then measured in the culture media. Principal component analysis (PCA) showed a clear separation of the LPS-treated samples from untreated samples and hypoxic samples (Fig. 2a). LPS treatment resulted in an increase in all cytokines/chemokines with the exception of IL-4 and MDC (Fig. 2b). Interestingly, a further separation of the LPS group into two subgroups was seen (Fig. 2a). No clear separation of the hypoxia-treated and untreated samples was observed.
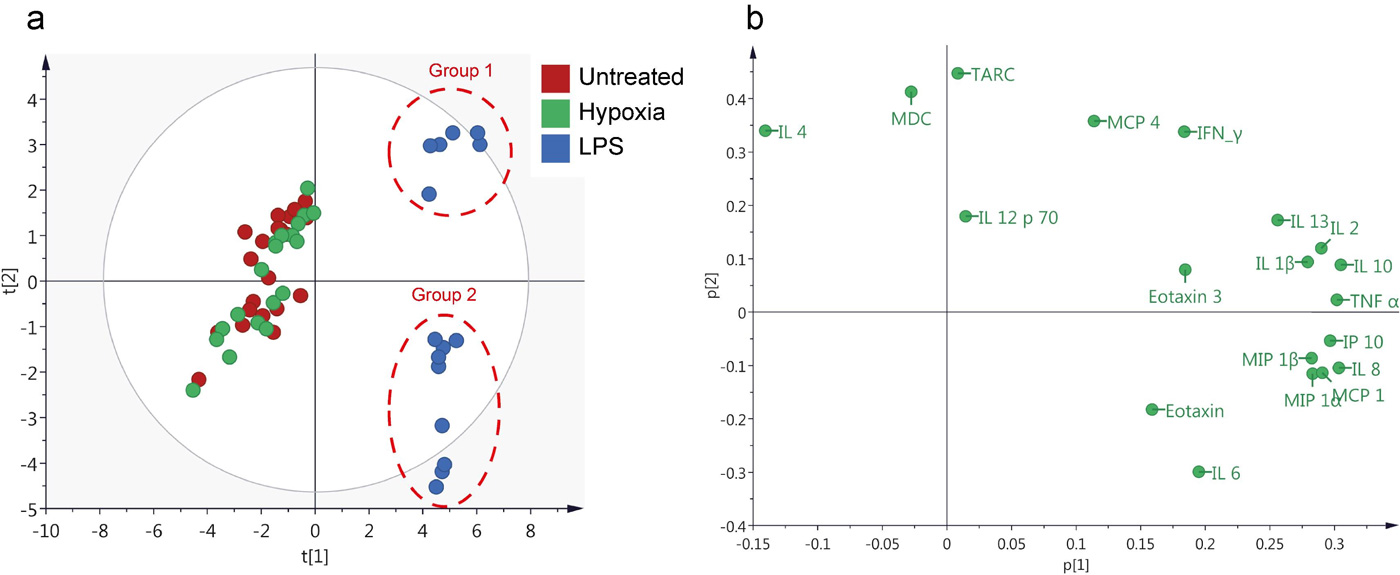 Fig. 2. Principal Component Analysis (PCA) analysis of cytokine and chemokine levels (Sandstedt, Joakim, et al., 2019).
Fig. 2. Principal Component Analysis (PCA) analysis of cytokine and chemokine levels (Sandstedt, Joakim, et al., 2019).
To confirm these findings, two-way ANOVA was carried out for each of the included cytokines/chemokines (Fig. 3). Taken together, the LPS group had a significantly higher production of most of the analyzed cytokines/chemokines compared to control samples and hypoxia-treated samples. Only production of IL-4 was significantly lower in the LPS group compared to the two other groups. No significant differences were observed between the control samples (no LPS, normoxic conditions) and hypoxia-treated samples.
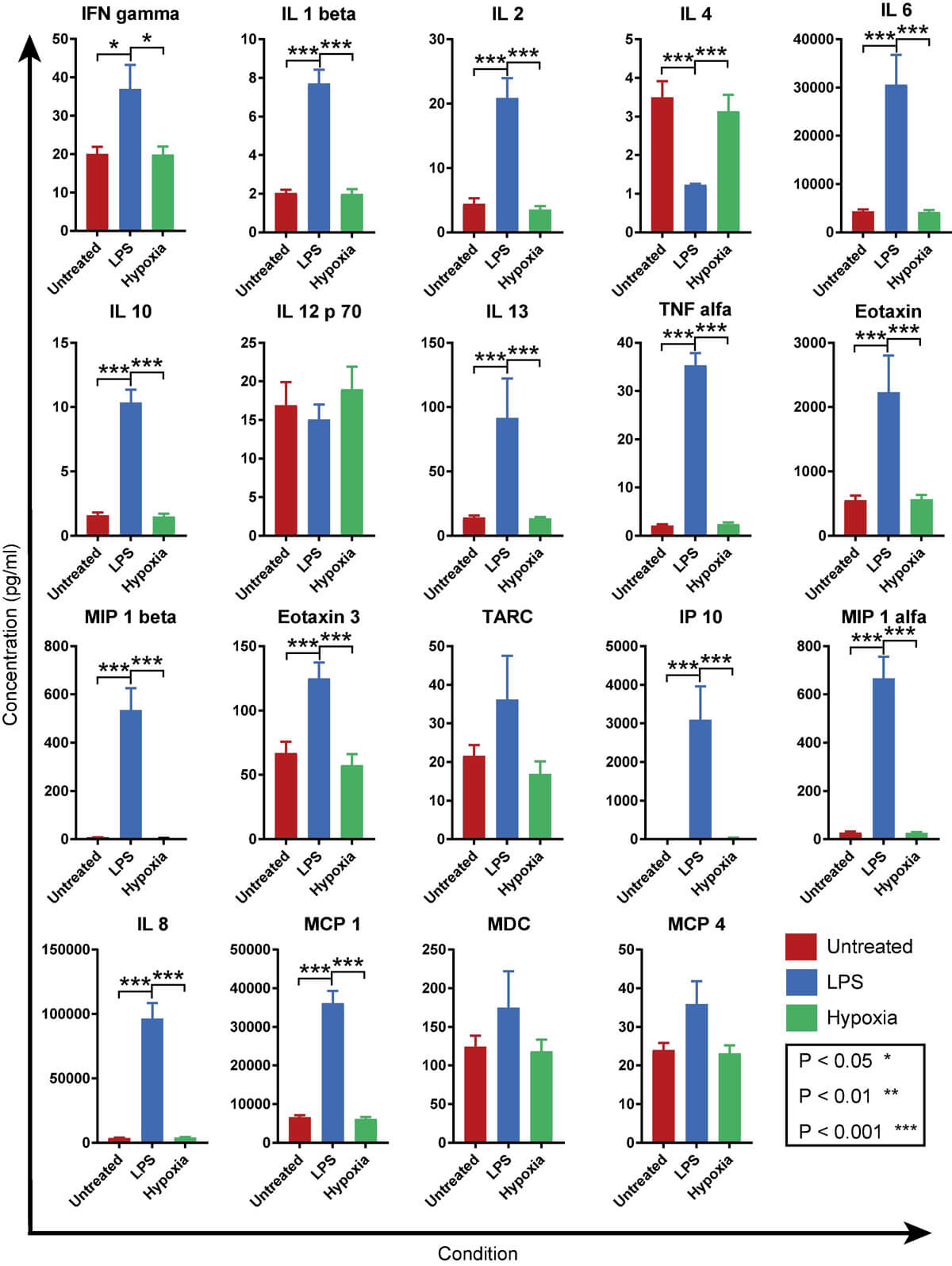 Fig. 3. Graphs showing the mean expression levels for different conditions (Sandstedt, Joakim, et al., 2019).
Fig. 3. Graphs showing the mean expression levels for different conditions (Sandstedt, Joakim, et al., 2019).
Yes, we have both sexes.
We strongly encourage using the recommended media and culture conditions as they have been optimized for cell growth.
The standard concentration used for subculturing is 0.25% Trypsin-EDTA. Yes, Trypsin-EDTA containing phenol red can be used as it does not interfere with trypsinization.
Media should be changed every 2-3 days or as specified within the recommended growth conditions.
Human cardiac fibroblasts are non-muscle cells found in the heart that play a crucial role in maintaining the structural integrity of the heart. They are responsible for producing extracellular matrix proteins, regulating cardiac function, and responding to injury or stress.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Satisfied
The cells arrived in great condition and were easy to culture. They have been performing well in my experiments and I have been able to obtain reliable results.
2 Aug 2023
Ease of use
After sales services
Value for money
Write your own review
